danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
Danial, Thank you for all the awesome insights so far.
I am super curious about the recent video of Robert Murry using a ceramic separator would be interesting to see if a dendrite could ever plate its way through that structure.
I have also been thinking about applications for even a ZnBr2 cell with high self discharge and i think it would be perfect time shifting power.
In New Zealand and we have 15 minute power pricing so in theory you could build a battery, buy power when its cheaper and use it when its quite expensive. during the day it could charge from solar for the evening peek usage. and again charge over night from very cheap renewable (We have lots of geothermal and hydro) for the morning peeks.
Great work, i cant wait to get my hands on some supplies and have a go my self.
Thanks for your message and kind words!
I would certainly want to try a ceramic separator sometime, if just to see how it does in terms of dendrites and internal resistance, but sadly ceramics require more equipment than I have or can procure in the small space I have available. Working with them - cutting, forming, heating, etc - is something my small apartment cannot handle.
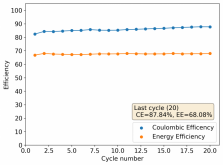
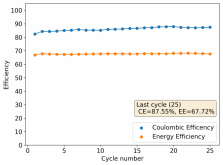
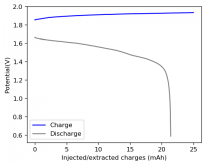
About self-discharge, this is hardly an issue in a battery like the one showed in Rob's latest video on the subject. The main issue in this case is the energy efficiency of the battery, which for a configuration like the one he showed is pretty dismal (<15%). This is due to both the distance between the electrodes and the ceramic separator. A battery where you need to put in 10Wh to get out 1-2Wh is hardly practical. To get to decent energy efficiency values (>60%) you really need to do a lot of work to reduce internal resistance, maximize cathode surface area, etc. In that case - with a more engineered battery - what you propose might be a very interesting use case.