Been mucking about with this again and thought I'd share the recent learning... made up a batch with 6 cups of water, 6 tsp
sodium Polyacrylate, and a 1/4 cup of isopropyl alcohol (should have used a 1/2 cup, when frozen the result is a bit stiff). No dye, just in case it leaked.
Then I bagged it using the wife's vacuum bagger. No vacuum just used the sealer function. Let the crystal absorb/swell overnight, then using a 1/4 cup baking measure put 1 cup in, then sealed, then another cup, sealed, etc.
Basically, I wanted a row of popsicle-like tubes as a single large bag as it melted it would all flow to the bottom of the bag. The concept semi-worked, when melted the top half sinks to the bottom half, couldn't get them tight enough to not collapse. The seams are big enough that it folds nicely in the freezer.
Then I put some duct tape around a couple of tubes so I could alter the height - but that plan didn't work out. |  |
The Test
I put the little 1-cup pouches I'd made last year in the front pockets of a fishing vest. The whole back unzips for cooling...but there's an inner mesh so it makes a really big pocket and that's where the ice-tubes went.
The first concern I had was that it would be too cold. But with a knit shirt it felt okay. The back felt cool, but the front felt warm.
I was also concerned that the cold and humidity it would cause the back to sweat, I did see some of that, but it was very minor.
The Tube shape is perfect for going across the upper back - makes good contact when pulled tight. The little pouches in the front pockets didn't feel like they were doing anything.
Overall the vest pockets just didn't work. They were too deep and everything wanted to sink to the bottom. It's subjective, but the cooling felt best high on the chest and back. In retrospect, your skin probably has the best heat transfer ability where your shirt gets soaked with sweat first.
To keep the back "tubes" from sliding down I tied a piece of rope around midway on the vest to try and hold them higher on the back. Looked goofy, but not only did it hold it up, the cooling vest seemed to work - I spent about an hour cleaning the garage (so still in shade) and hadn't broken a sweat. I know, doesn't sound like much, but an hour usually leaves me crawling back in begging for air conditioning. There was some melting, but a lot of ice left. Probably would have lasted 2 hours. Not sure how well I would have done in direct sunshine.
Lessons Learned
- They work! Time to take the summer back!
- Even though I was carrying 8 cups of frozen water (4 lbs), the weight didn't feel bad.
- They need to be high and snug (contact anyway), can't just be something sitting in your pockets.
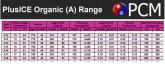
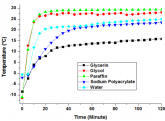
- You don't need to make your own gell packs, turns out there are all sorts of seriously inexpensive gell packs in pretty much every shape (images are amazon links) for medical reasons. Some can be put into the microwave to double as "heating pads" (learned this recently while looking for a new heating pad (don't ask ;-).




- large pouches (like the Remedy above) slouch when they melt.
Next Steps
To go farther with the test unit I'd need some way to control the adjustments, the rope-trick is pretty weak. The vest isn't well balanced with the ice either, probably because I had 6 cups in the back and two in the front. I had no way to keep the front ice packs where I wanted and it didn't feel like they were providing anything. ;-)
I'm thinking about getting a professionally made version. If anyone has recommendations let me know. What I think I'm looking for is:
- Something that has adjustments, getting it snug seems to be key to keeping cool.
- Definitely ice. Air or "wet evaporation" just won't work well here as it is too humid.
- Possibly tube shapes on the back and squares or triangles on the front so the wife can use it too.
Amazing that the gel packs and vests are inexpensive, but something like this is expensive: