inspectorrgadget
New Member
- Joined
- Mar 22, 2021
- Messages
- 15
I created high-conductivity battery terminal studs for my SOK battery. I am willing to make more of them so others can get something better than a steel bolt to conduct electricity in their RV or solar setup.
The studs I made are metric M8 x 1.25 in high-conductivity brass.
Originally, my SOK-brand LiPoFe batteries used stock metric M8 x 1.25 bolts that threaded into the batteries. I feared that constantly removing and replacing the steel bolt into the alloy threaded hole might wear out the battery connection. So I converted to a studs-and-wing nuts arrangement in order to attach the cables to the batteries. In thousand of miles, the wing nuts have never come loose, and I don't need to go searching for a wrench each time I want to take the batteries out of my RV.
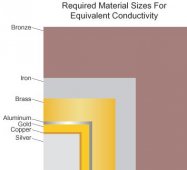
To enhance conductivity, decided to upgrade the battery studs to metric M8 brass. Because brass is a mixture of copper, iron, and often other metals (such as boron or nickle), most brass alloys do a better job of carrying current than do most types of steel. McMaster-Carr sold me a 1-meter long M8 threaded brass rod. One of the attributes listed in this rod's specifications is being electrically conductive:
https://www.mcmaster.com/90162a080/
Brass Threaded Rod
M8 x 1.25 mm Thread Size,
Item 90162A080.
I used a Skillsaw and a file to make four 1.5 inch-long studs for myself. Doing so took 6 inches off the 39-inch rod. Each of my studs is just under 1.5 inches long.
I have 33 inches of M8 threaded brass rod remaining, which I would sell to anyone who makes a decent offer for the leftover rod. Or, I could make 22 more pieces for any others out there who might also want some brass stud terminals for their SOK batteries.
I'd charge $3 bucks for a 1.5 inch stud, plus shipping.
If anyone is interested, DM me on this forum.


The studs I made are metric M8 x 1.25 in high-conductivity brass.
Originally, my SOK-brand LiPoFe batteries used stock metric M8 x 1.25 bolts that threaded into the batteries. I feared that constantly removing and replacing the steel bolt into the alloy threaded hole might wear out the battery connection. So I converted to a studs-and-wing nuts arrangement in order to attach the cables to the batteries. In thousand of miles, the wing nuts have never come loose, and I don't need to go searching for a wrench each time I want to take the batteries out of my RV.
To enhance conductivity, decided to upgrade the battery studs to metric M8 brass. Because brass is a mixture of copper, iron, and often other metals (such as boron or nickle), most brass alloys do a better job of carrying current than do most types of steel. McMaster-Carr sold me a 1-meter long M8 threaded brass rod. One of the attributes listed in this rod's specifications is being electrically conductive:
https://www.mcmaster.com/90162a080/
Brass Threaded Rod
M8 x 1.25 mm Thread Size,
Item 90162A080.
I used a Skillsaw and a file to make four 1.5 inch-long studs for myself. Doing so took 6 inches off the 39-inch rod. Each of my studs is just under 1.5 inches long.
I have 33 inches of M8 threaded brass rod remaining, which I would sell to anyone who makes a decent offer for the leftover rod. Or, I could make 22 more pieces for any others out there who might also want some brass stud terminals for their SOK batteries.
I'd charge $3 bucks for a 1.5 inch stud, plus shipping.
If anyone is interested, DM me on this forum.