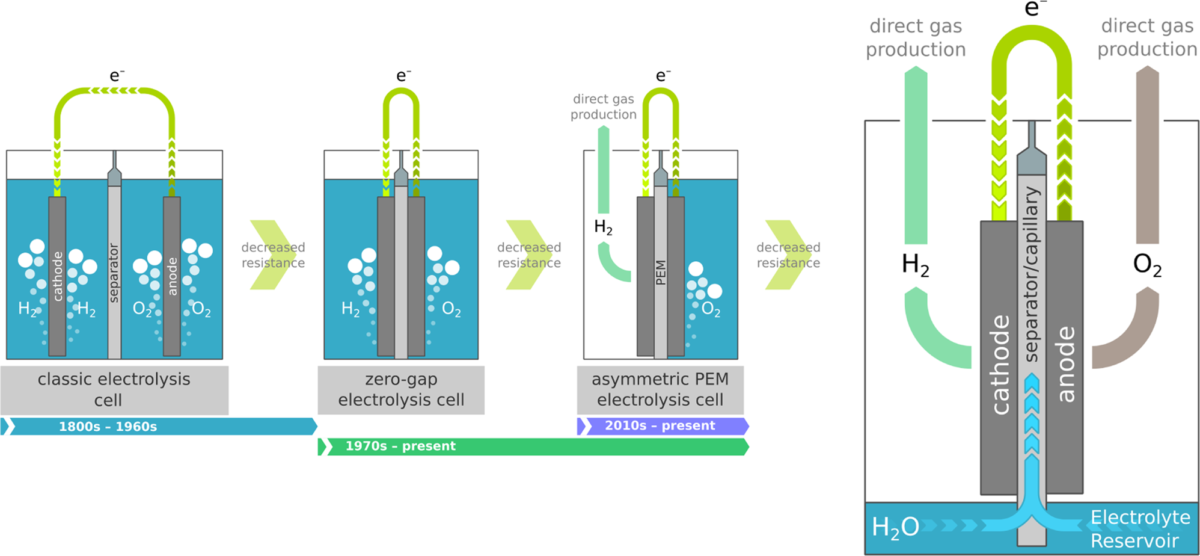
Plasm
New Member
- Joined
- May 11, 2022
- Messages
- 10
Ah okay, wasn't totally sure ^^Yeah, “next door” was tongue-in-cheek but it’s still very cool.
Have you seen this?
I haven't seen the video till now. He has a cool project, especially the energy recovery on the heat pump is very advanced. Energy recovery of ventilation/heating systems is just getting popular in the last years. His hydrogen storage sounds comparably low pressure, maybe the tanks aren't rated for really high pressure, but the amount of tanks he got already provide a nice amount of power. 1.5 MWh if I calculated correctly from his 40 gallons of gasoline example. I'm kind of jealous of his property. I couldn't fit any of that big tanks and am pretty sure you need a permit in Germany to put one in your yard.