@HighTechLab @Will Prowse - A different application going down to zero may help explain things.
Draining a LifePo4 down to zero is not an uncommon occurrence with motorcycle starter-type LFP's. These are typically high-rate cells. Brands like Shorai, Anti-Gravity, Earth-X, you name it. Some come with BMS, some don't. In fact my first ridiculously expensive LFP bank was made from these - and I'm not a motorcycle rider!
But the fact is that some may forget to turn off parking lights, leave their glove-heaters on and wake up the next morning to find their $350 4ah lifepo4 dead at zero volts. Revival is mandatory when you are 600 miles from home.

Long story middlin' short:
1) To recharge, you must get to it in time. We know that leaving cells at zero volts start to degrade chemically, but apparently not overnight. Or maybe in a few days. I don't have an exact timeframe, but the sooner the better.
2) To recharge from zero volts means you must use very very little current to get out of the steep discharge knee. Why? Aside from the whole dendrite thing, there is so much voltage potential at the charger, that running normal current can have the ability to blow out active material into the electrolyte, contaminating it.
In addition, what can also happen with too much current, is for lack of a better name, an "ion storm". What this means is that the lithium-ions can't intercalate fast enough and have no where to go. When they can't intercalate, they produce heat and possible secondary reactions. Which is bad.
One commercial charger manufacturer for lithium bike batteries knows this. When going into it's "save" mode, it applies very little current and won't start to charge with it's normal rated current until out of the discharge knee, and the cells have reached nominal 3.2v/cell.
MY TEST:
So other than it making sense, I wanted to SEE this so-called "ion storm" in process. How do I go about that without using my expensive cells as a test bed?
Answer: programmable hobby charger, and um, high-quality solar walkway light LFP batteries! Use high-quality of course.

Seriously, I did the best I could. I went with Westinghouse cells. 500mah capacity LFP AA's.
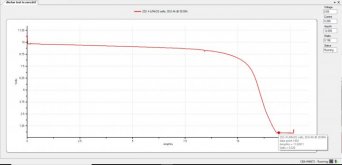
Used the hobby analyzer to drain them to zero volts. A few times when they recovered a bit.
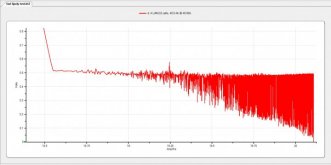
HAMMER TIME! Being safe about it with goggles, outdoors etc, I hit them up at 1C when they were at zero volts.
What I witnessed was an immediate rise in voltage (to be expected), but then a major stall! They barely got off the ground, and for all intents and purposes, no charging whatsoever was taking place. But they were being hammered at 1C nonetheless. Wouldn't rise. What I *think* I'm seeing here was the so-called "ion storm", where basically the ions were just in the mosh-pit.

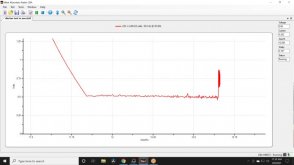
Allowing them to rest, and using very little current, as little as I could provide until they reached 3.2v allowed me to hammer them at 1C ! Not that walkway solar lights like this...
So maybe there is a larger test looming:
Drag a known capacity big lifepo4 down to zero volts. Do not let sit longer than overnight that way.
Charge at something like 0.01C until the nominal voltage is 3.2v/cell
Change charge amperage to your desired nornal rate, and test for normal capacity at your normal lvd.
This way, you may not see any capacity loss. Or maybe you will, but I don't know the percentage. I just know that one of my favorite bike-battery chargers seems to follow this procedure, which is not an uncommon occurrence for bikers using LFP starter batts. Or, for all I know, this is only applicable to high-rate cells.