It also seems that I have been underestimating compression in my cells by a very large amount when using fiberglass separators. I measured the inner cell height as a function of the distances between the caps of the swagelok cell on the outside and found out that I am actually working with substantially smaller cell gaps than I anticipated, as I am able to compress the device a lot when the cell is closed (fiberglass and carbon felt both compress a lot). This means that I have been working with cells that are actually only around 2.5-3mm tall, which means that I have been measuring and aiming for energy densities in the 40-50Wh/L range, while I previously thought the cells were around 5.8mm tall, as this is what I measured with a caliper when I took apart the devices, but compression makes the devices way shorter.
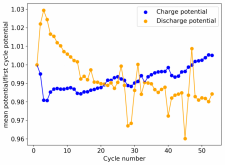
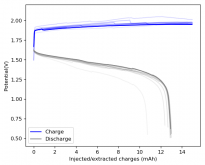
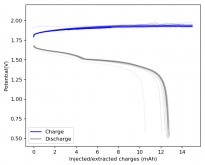
I put together a cell with double the amount of separator, in an inverted configuration using a GFE-1 cathode pretreated with a 10% TMPhABr solution, using a 4.2M ZnBr2 solution with 1% TW20 which gave me a compressed height of 4.8mm (energy density of 29.81Wh/L). Note that I used a much more concentrated electrolyte because I wanted to try the straight electrolyte from a ZnBr2 synthesis from sodium sulfate and zinc sulfate, the approximate concentration was obtained by measuring the density using a pycnometer. The results are below.
View attachment 29488
View attachment 29489
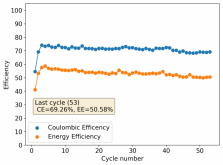
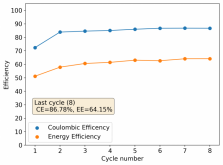
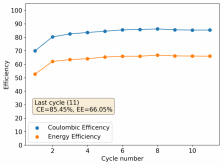
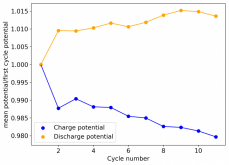
I stopped cycling the cell here because I wanted to open it to see if any deterioration had happened up until this point - given the higher concentration of ZnBr2 - I saw no evidence of dendrites though. The CE is acceptable at 86.78%, and the EE is the highest within the cells I've measured in inverted configurations at 64.15%.
During the next few days I will put together another cell in this configuration and run it for longer, to see if I can reproduce the above results and see whether these taller cells can produce better results. So far no cell has been stable enough to last more than 100 cycles, we'll see if this one is the charm ?