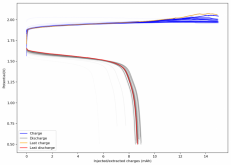
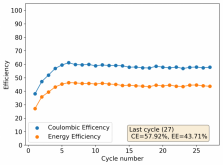
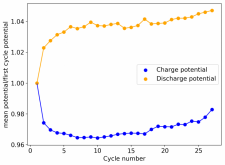
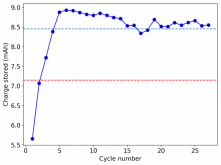
Thanks for posting ? Glad to see someone else joining!
I would love to see what performance you get using a lower resistance electrode like the type I described above. If you are not using CAPLINQs 200 ohm plastic, I doubt you are optimized. They are currently making the best conductive plastic on the market (to my knowledge).
I don't use any conductive plastics, the Swagelok cell I use - described in my last blog post in chemisting.com - uses graphite electrodes and a GFE-1 carbon felt electrode, the resistance of the graphitic felt electrode is below 10 ohm, so it is much more conductive than these plastics (almost an order of magnitude more than the ones you mention). Conductive plastics are not going to work well, even at 75ohm, that is still way too high. If all electrodes are graphitic felts or clothes, the conductivity is going to be way better. You'll notice no papers use conductive plastics, precisely for this reason. Any configuration that expects to work with good energy efficiencies at current densities > 10mA/cm will use graphitic felts and/or cloths.
About shorting, I know this can help eliminate dendrites and "reset" the chemistry, but it is my goal to try to come up with a chemistry that does not require this short circuiting step as this is a significant limiting factor of this chemistry's implementations up until now. There are also some chemical processes - such as hydrogen evolution when the top electrode is the zinc electrode - that are irreversible, the battery will never recover from these processes.
The Princeton papers on minimal architecture ZnBr2 batteries acknowledge that large scale setups will require inverted geometries - Zn electrode at the bottom - due to this reason. Iron impurities also generate a lot of issues in long term cycling when the ZnBr2 source is not high purity (>99.98%), this has been a big issue for commercial ZnBr2 flow batteries.
About your recommendations:
1. I think it is well established that anything between 1.5-3M ZnBr2 will work fine. I have seen significant difference in dendrite formation between concentrations though, so it is probably worth it to run experiments to determine cycle life at different ZnBr2 concentrations. The spacer or separator design will also depend a lot on your ZnBr2 concentration. It is likely better to commit to a ZnBr2 concentration, say 2M, and optimize the spacer or separator design based on that. Separator materials, cell design, etc, will all play a role in this.
2. The problem with these sequestering agents is their solubility in the electrolyte. At a 2M ZnBr2 concentration these are basically insoluble in the electrolyte. In a flow cell this does not matter because you have an anolyte that contains only the BSA but in a static cell, the insolubility of the BSA creates a lot of problems with the kinetics of the sequestering and irreversible processes in the device.
In batteries with a normal configuration - Zn electrode on top - hydrogen evolution damages the battery, as H2 escapes and makes the electrolyte more basic -, in an inverted configuration - Zn electrode at the bottom - the BSA migration from top to bottom creates a significant issue as the BSA gets "lost" in the middle of the battery. The Trimethylphenylammonium bromide (TMPhABr) I used, was the best I could find to reduce these issues, but they are still present and seemingly quite insurmountable for any BSA of this type. The best option might be to actually functionalize a graphitic felt with a sequestering compound so that you can have effective sequestering without needing anything to dissolve or migrate in the electrolyte.
3. These additives can be quite critical, I wouldn't give them lower priority than the BSA. Through my research I believe I've established quite conclusively that 0.5-1% Tween 20 is effective at reducing dendrites. I've also established that PEG-200 reduces conductivity too much to be useful and conductive salt additions such as NaBr or NaCl greatly increase dendrite formation due to their effects on Zn ion migration.
I believe that perhaps the most important part is to define an experimental setup that we can all use and reproduce because while everyone is using different electrodes, geometries, etc, it is quite difficult to share and reproduce results. I believe for initial small scale experiments, a setup like mine has big advantages as everyone can pretty much build it and guarantee we all share the same geometry and measuring instruments. Swagelok cells are a standard in battery research for small scale experiments and the open source galvanostat I used provides everyone with the ability to measure charge/discharge curves and perform other standard experiments. The total cost of the testing setup is probably around 400 USD (swagelok cell, electrodes, etc). If anyone is interested in how to get everything, just let me know and I'll guide you as best as I can.