danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
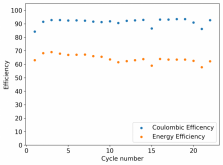
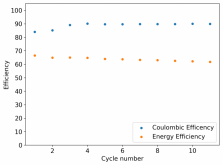
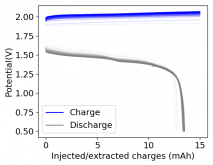
I ordered some Sodium Bromide and Zinc Sulfate in order to test an electrolyte comprised of Zinc Bromide, saturated with Sodium Sulfate plus 20% PEG-200. Sodium sulfate is known to reduce the edge formation of Zinc dendrites (see here) so this seems like a nice experiment to test a much cheaper electrolyte that can also help with the formation of dendrites. The electrolyte is much cheaper because both NaBr and ZnSO4 are available in kilogram quantities for low amounts of money (compared to Zinc bromide, which is much more expensive at small scales).
In order to prepare the electrolyte I plan to prepare a solution with 6M NaBr + 3M ZnSO4 + 20% PEG-200, this will give me the 3M ZnBr2 I need but will be above the solubility limit of NaSO4 at ambient temperature, so I will filter out the solution to remove the precipitated NaSO4. This should serve to reduce the series resistance of the cell and hopefully the dendrites as well. These are experiments for next week though, in the meantime I'll keep on testing my current electrolytes made of 3M ZnBr2.
In order to prepare the electrolyte I plan to prepare a solution with 6M NaBr + 3M ZnSO4 + 20% PEG-200, this will give me the 3M ZnBr2 I need but will be above the solubility limit of NaSO4 at ambient temperature, so I will filter out the solution to remove the precipitated NaSO4. This should serve to reduce the series resistance of the cell and hopefully the dendrites as well. These are experiments for next week though, in the meantime I'll keep on testing my current electrolytes made of 3M ZnBr2.
Last edited: