danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
Welcome to my adventure building a Zinc-Iodine battery
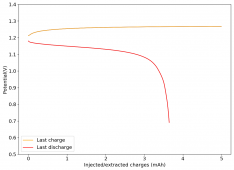
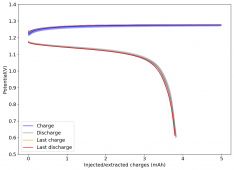
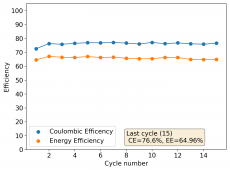
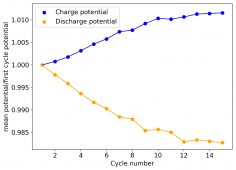
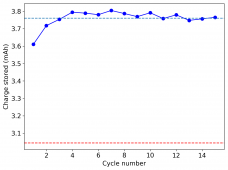
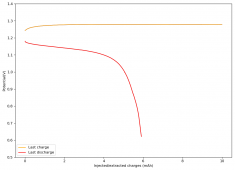
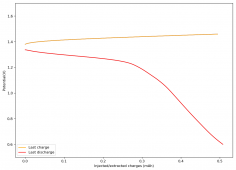
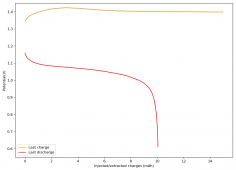
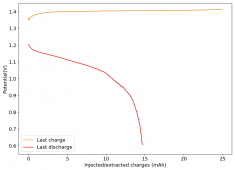
All experiments carried out from #100 are now shared publicly (https://www.dropbox.com/sh/67hdgpm5lijt5s1/AACS0gjDSYHZny_tLSfXXmfza?dl=0). The experiment_summary excel file contains a summary of all important variables in the experiments that have been ran, the analysis_images folder contains processed analysis results with the commonly expected curves (charge/discharge curves, capacity, voltage evolution, etc), the raw_data_files folder contains all the raw data from the experiments, in case you want to carry out your own analyses.
All experiments are carried out using a Swagelok cell with a 0.5 inch diameter Swagelok cell, vertical cross-section showed below:
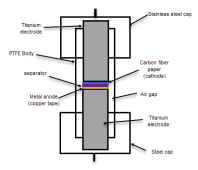
Titanium electrodes are sanded using 240 paper between experiments. The Swagelok cell and electrodes are always cleaned with ethanol - which fully dissolves iodine - and water between experiments. All anodes/cathodes/separators used between experiments are new, most materials are cut to size using a 0.5 inch hole puncher.
Electrochemical measurements are carried out using an open-source USB potentiostat-galvanostat built using the gerber files and code provided here. The PCB was manufactured through PCB-way. The python program included in that article is also used to carry out the electrochemical experiments.
-------------------------------------------------------------------------------------------------------------------------------------------------------------
Original #1 post below:
Now that my adventure with Zn-Br batteries is over, I have decided to move to the Zinc-Iodine chemistry. I will start by attempting to reproduce this paper, which uses a high surface area, highly conductive carbon electrode, a zinc sulfate electrolyte and a zinc anode. They achieve a highly stable battery due to the confinement of the iodide ions within the activated carbon nano structures.
I have elemental iodine, a GFE-1 high surface area graphitic felt, zinc sulfate and zinc metal, so I'm going to try and load the GFE-1 cathodes with iodine and see how it goes ?
All experiments carried out from #100 are now shared publicly (https://www.dropbox.com/sh/67hdgpm5lijt5s1/AACS0gjDSYHZny_tLSfXXmfza?dl=0). The experiment_summary excel file contains a summary of all important variables in the experiments that have been ran, the analysis_images folder contains processed analysis results with the commonly expected curves (charge/discharge curves, capacity, voltage evolution, etc), the raw_data_files folder contains all the raw data from the experiments, in case you want to carry out your own analyses.
All experiments are carried out using a Swagelok cell with a 0.5 inch diameter Swagelok cell, vertical cross-section showed below:

Titanium electrodes are sanded using 240 paper between experiments. The Swagelok cell and electrodes are always cleaned with ethanol - which fully dissolves iodine - and water between experiments. All anodes/cathodes/separators used between experiments are new, most materials are cut to size using a 0.5 inch hole puncher.
Electrochemical measurements are carried out using an open-source USB potentiostat-galvanostat built using the gerber files and code provided here. The PCB was manufactured through PCB-way. The python program included in that article is also used to carry out the electrochemical experiments.
-------------------------------------------------------------------------------------------------------------------------------------------------------------
Original #1 post below:
Now that my adventure with Zn-Br batteries is over, I have decided to move to the Zinc-Iodine chemistry. I will start by attempting to reproduce this paper, which uses a high surface area, highly conductive carbon electrode, a zinc sulfate electrolyte and a zinc anode. They achieve a highly stable battery due to the confinement of the iodide ions within the activated carbon nano structures.
I have elemental iodine, a GFE-1 high surface area graphitic felt, zinc sulfate and zinc metal, so I'm going to try and load the GFE-1 cathodes with iodine and see how it goes ?
Last edited: