danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
I've been trying to increase the cycling stability of the batteries, as most of the batteries I made were either dying due to shorts after 5-10 cycles or their capacity was fading quickly after the first 10-20 cycles.
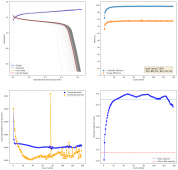
I tested a battery using a CCP cathode (150um) with a Whatman 42 filter (200um) paper as separator and a Zinc anode. The electrolyte is made from 15m ZnCl2 + 5m KI + 5m NaCl in white vinegar (4% v/v Acetic acid). The idea of the white vinegar is to increase ion mobility, since the dipole moment of acetic acid is larger than that of water. Note that the electrolyte itself is already very acidic (pH < 2), so the acetic acid is all protonated under these conditions and merely provides a higher dipole moment media.
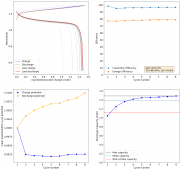
I wanted to test stability. This means reaching a higher cycle number quickly, for this reason, the battery was charged to 1.3V at 10mA, discharging to 0.5V. Previous attempts had shown poor results under these conditions. The results up until now are below (first 100 cycles):
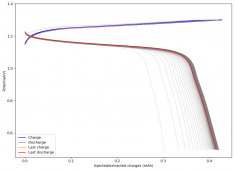
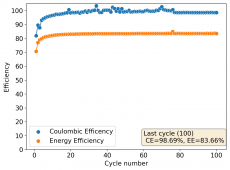
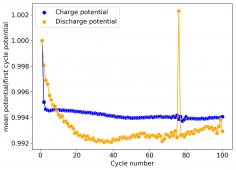
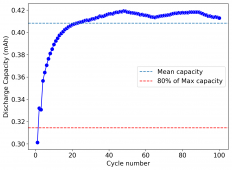
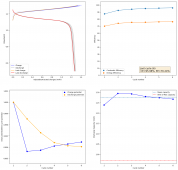
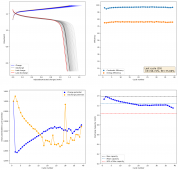
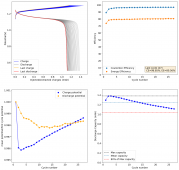
The CE is stable and mostly above 98% and the EE is above 80%. So far capacity has been quite stable, although it is clear that some instability still remains. Since the battery is being charged to a low potential at a relative high current density (7.75mA/cm2), the energy density is quite low, currently at around 9.66Wh/L. I want to be able to reach a cell that is stable for >500 cycles before I aim for higher capacity, as higher capacity and densities are meaningless if a cell is simply unstable.
The achievement of a CE > 98% is already a significant improvement from the original paper. Both the use of vinegar and the use of a Zn metal anode (instead of the graphite anode of the Swagelok cell) have been critical in the improvement of the battery.
I tested a battery using a CCP cathode (150um) with a Whatman 42 filter (200um) paper as separator and a Zinc anode. The electrolyte is made from 15m ZnCl2 + 5m KI + 5m NaCl in white vinegar (4% v/v Acetic acid). The idea of the white vinegar is to increase ion mobility, since the dipole moment of acetic acid is larger than that of water. Note that the electrolyte itself is already very acidic (pH < 2), so the acetic acid is all protonated under these conditions and merely provides a higher dipole moment media.
I wanted to test stability. This means reaching a higher cycle number quickly, for this reason, the battery was charged to 1.3V at 10mA, discharging to 0.5V. Previous attempts had shown poor results under these conditions. The results up until now are below (first 100 cycles):




The CE is stable and mostly above 98% and the EE is above 80%. So far capacity has been quite stable, although it is clear that some instability still remains. Since the battery is being charged to a low potential at a relative high current density (7.75mA/cm2), the energy density is quite low, currently at around 9.66Wh/L. I want to be able to reach a cell that is stable for >500 cycles before I aim for higher capacity, as higher capacity and densities are meaningless if a cell is simply unstable.
The achievement of a CE > 98% is already a significant improvement from the original paper. Both the use of vinegar and the use of a Zn metal anode (instead of the graphite anode of the Swagelok cell) have been critical in the improvement of the battery.