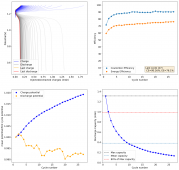
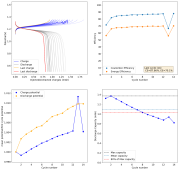
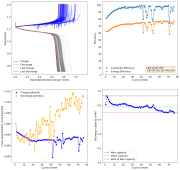
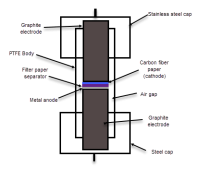So I decided to not run this experiment, as getting long term cycling results of batteries at low current densities is not practical, it would take months to get hundreds of cycles. The lowest current that I believe is practical to gather results for me is around 2.0mA/cm2. This means I will need to start testing solutions to alleviate the dendrite problem in the WiS electrolyte. Here are some of the additives that have been used in the literature to reduce dendrites with some success (some which I tried with the Zn-Br cells with some success):
- Boric Acid
- Thiourea
- Cetrimonium bromide
- Sodium dodecyl sulfate
- PEG-200
- Tween-20
I have cetrimonium chloride, which has been tested to reduce dendrites in lithium but not zinc batteries, so I will try this one first. I also have Tween-20, which I used in Zn-Br batteries successfully, so that will be my next try.
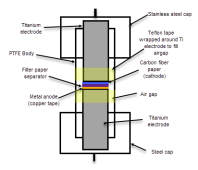
The first attempt will be a cell with a 15m ZnCl2 + 5m KI + 0.00375m cetrimonium chloride (around 1200ppm) in distilled water. The cell uses a zinc anode, W42 separator and a Spectracarb 2050A-1050 cathode, using the Ti electrodes in the Swagelok cell. Experiment will be to charge to 1.3V at 2.5mA, discharge to 0.5V.