I didn't mean it as a physically strong resisting penetration or increasing distance as when you went to 6 layers.
Dendrites probably grow one atom at a time, probably following the same rules as the snowflakes. That the dendrites beeline for the other electrode currently says conditions and crystal geometry favor that growth to occur that way.
I meant it as a dendrite "DNA alteration". Something in the separator or solution alters the "skeletal growth/shape" of the dendrite. If it inhibits "straight-line" growth or causes their shape to change such that they don't reach out towards the far electrode or the growth points to be redirected back rather than through then its a win. For example, if there was something in solution that latched on the vertex such that the dendrites went sideways than outwards or like the flat edge in the snowflake video causing no growth in that direction.
The Sn approach I mentioned does something like this, when the dendrite reaches the Sn layer, it is no longer thermodynamically favorable for the dendrite to grow.
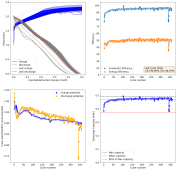
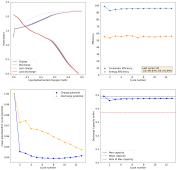
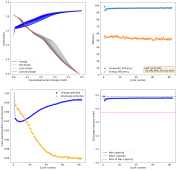
Dendrites grow mainly because there is a gradient of Zn ion concentration going from the electrode to the bulk of the solution. As Zn ions are plated very fast at the electrode surface, they are depleted quickly, anything that protrudes into the solution requires shorter diffusion of Zn ions, so they get plated there faster. The more a dendrite grows, the faster access it has to the bulk concentration of ions in solution and the more surface area it has to branch and spread. It is a vicious cycle that magnifies the smallest protrusions in a zinc surface, growing them all the way to the cathode, shorting the battery.
There are some approaches to prevent this with additives, surfactants like polysorbate, laureate and cetrimonium, will in theory preferably cling to dendrites instead of the electrode surface, passivating them and preventing dendrite growth to increase. The problem is that they also affect the chemistry of the solution and, I've noticed, can encourage dendrites in some cases (when I put cetrimonium chloride in my devices, they form dendrites much faster, meaning cetrimonium chloride must adhere to the Zn surface instead of the dendrites in my particular case).
There are also approaches that modify the anode, for example by plating it with Cu or Sn through exposure to Sn or Cu solutions.
Using CuSO4 plating solution -
https://www.mdpi.com/2079-4991/11/3/764
Using SnCl4 plating solution -
https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.201906803
These lead to the formation of more stable metal alloys that do not favor dendrite structures as the energy for plating on them is lower than in pure Zn dendrites.
I have tried these two approaches with no success.
My dendrite issues notably happen much faster than normal Zn dendrite related failures in the literature and do seem to be located directly around my separator edges, it might be a feature of my fiberglass separator architecture that facilitates their growth around these points. I might need to get a new fiberglass separator with different porosity to see if this is the case.
I can also try a separator-less approach using teflon rings, to see if this is the case.