danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
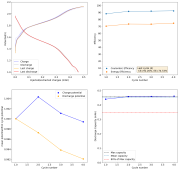
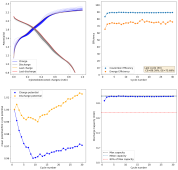
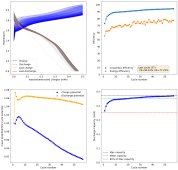
I ran this experiment for 43 cycles (Exp31b, results below), charging to 1.5mAh at 5mA/cm2. The CE remains high, EE remains high, capacity was 1.38mAh on average. Energy density at this capacity, given total device volume,, is 16Wh/L.
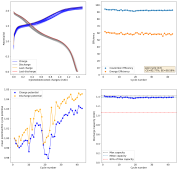
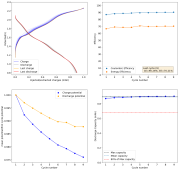
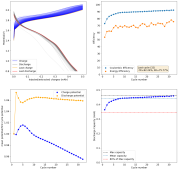
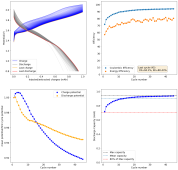
I have now done another single conditioning cycle, charging to 2.2V at 5mAh/cm, then setting the battery to 2.2V until the current dropped to 1mAh/cm2, then fully discharging. I am now cycling by charging to 2.5mAh at 5mA/cm2 and discharging to 0V.

I have now done another single conditioning cycle, charging to 2.2V at 5mAh/cm, then setting the battery to 2.2V until the current dropped to 1mAh/cm2, then fully discharging. I am now cycling by charging to 2.5mAh at 5mA/cm2 and discharging to 0V.