chrisski
Solar Boondocker
- Joined
- Aug 14, 2020
- Messages
- 5,471
Any tips for measuring Battery resistance? Device? Only when top balanced or should this even matter"
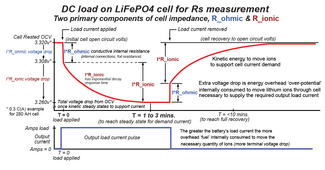
I have a battery capacity tester that gives me a reading, but can quickly climb as the test goes on. I have the topband 25 ah lithium cells from battery hookup. When I hook my DL-24P capacity tester to these batteries after top balancing, the batteries measure 3 mΩ or 4 mΩ, which matches what is advertised. Almost immediately resistance will rise to 5 mΩ and within minutes be 17 mΩ and over the course of a 5 amp 5 hour discharge cycle will rises as high as 50mΩ
I'm trying to match my batteries as much as possible, and don't know what the standard is since I expected resistance to be the same.
I have a battery capacity tester that gives me a reading, but can quickly climb as the test goes on. I have the topband 25 ah lithium cells from battery hookup. When I hook my DL-24P capacity tester to these batteries after top balancing, the batteries measure 3 mΩ or 4 mΩ, which matches what is advertised. Almost immediately resistance will rise to 5 mΩ and within minutes be 17 mΩ and over the course of a 5 amp 5 hour discharge cycle will rises as high as 50mΩ
I'm trying to match my batteries as much as possible, and don't know what the standard is since I expected resistance to be the same.