davetelling
New Member
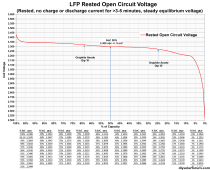
I am just starting out with a 280AH LiFePO4 battery, after having used some flooded lead-acid for a couple of years. I've looked online to see if I could find some info on how low the battery terminal voltage should drop under load, but haven't found anything other than generalities. With a fully charged battery, what should I expect to measure at the battery terminals when I connect a 100+A load? It seems to go lower than I would expect. The battery started at 13.38 volts. I turned the inverter on, set the heater load to high, and showed 135A draw from the battery. the voltage at the battery dropped to 12.5 volts after about 60 seconds, then after another 60 seconds, dropped to 12.48 volts. I turned the heater to the medium heat setting, and the current dropped to 85 amps, the battery voltage then rose to 12.7 volts. At this point, I watched the voltage for about 30 seconds, then shut everything off. The battery voltage rose fairly quickly to about 13.2 volts, then continued to rise to 13.26 volts after about 5 minutes. I went back inside, but noticed that the battery voltage was still slowing creeping upward.
So, does this seem normal? The only comparison I have is with two paralleled 85AH flooded lead-acid cells, and under about the same conditions, dropped from 12.7 to 11.8 volts with the same load.
So, does this seem normal? The only comparison I have is with two paralleled 85AH flooded lead-acid cells, and under about the same conditions, dropped from 12.7 to 11.8 volts with the same load.