Lithium is a great battery technology!
They last for many cycles, unlike lead the effect from Peukert's law (power available decreases based on discharge rate) is negligible, typically over 90% round-trip efficiency, they self-discharge very slowly, and don't lose power as quickly when they get cold.
But, there are a few things to be aware of with lithium chemistry that are discussed below.
LiFePO4 degrades when held at 100% charge indefinitely
This is called
capacity fade:
. |
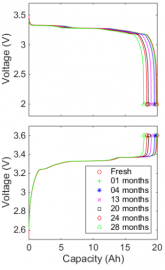
Charge discharge curve of cells stored at 100%
| Top is irreversible loss, bottom is reversible loss
|
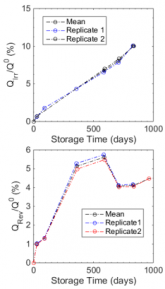
As you can see from the graphs above, a battery held at 100% charge would be down 10% capacity in 3 years. The way to not lose this capacity is to not store batteries for long periods at 100% charge. See the paper cited in the link above for more.
They have a "memory" affect that can strand capacity
Lithium has a small memory effect that builds up with micro-cycles
over a small voltage range.
During the course of a day the charging/discharging isn't enough
to impact them if they reach full charge.
But, if you don't commonly use your batteries and the BMS upper
limit is 90%, then they might be microcycling around that value
and that can strand capacity.
The memory effect can be erased with a full charge, but if it builds
up to where the effect voltage is greater than or equal to the full charge voltage you may lose capacity. | 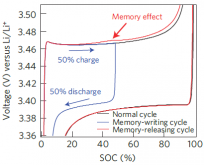 |
They shouldn't be charged when cold
Even charging just once at zero or below can permanently damage your
batteries.
Cold temperatures are known to be detrimental to the cells if they are exposed to charging. Cycling performance tests at varying temperatures showed the apparent existence of a threshold below which capacity fade with cycling suddenly accelerated. This threshold appeared to be above the temperature of 0°C often suggested as limit for recharging, but the data available was limited and the exact details of cell manufacture are likely to influence this value. <reference needed>
Overcharging & Float charging will damage them
Lithium batteries should not be "trickle" charged. There is no such thing a safe charging voltage that can be maintained continuously with lithium [
ref]. A lithium battery that is being held at an
elevated voltage with zero current flowing in has been overcharged and is getting damaged. Any voltage from 3.4V up can most definitely overcharge and damage a lithium cell.
High C-Rates will degrade them
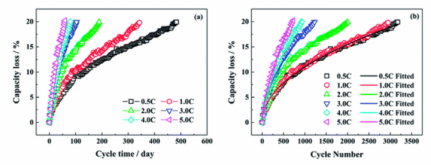
Heat degrades them