Probably, but then it would be best to not ventilate, just have a small few holes so things can equalize.
If I decide to go that route, the idea would be to use a heating pad to maintain at least 23C (or whatever) and use a fan, possibly connected to a vent/hose going outside, to blow in cool 65F air whenever temps climb past 27C (or whatever). There will only ever be ‘ventilation’ when the entire compartment starts approaching a danger zone (likely only because of sustained current draw).
18 °C is totally fine, it's actually better than 25 from what I saw.
I have one cell that appears to have taken significantly less charge to 3.65V at 18C but I only discovered that at the end of the capacity test.
Charged at 25C the cell actually delivered a hair over 280Ah, so now I need to circle back and repeat a test charging a 18C to understand whether the potential issue is ‘real.’
These grey market cells came so cheap, there is almost certainly
something wrong with them.
EVE’s specification only shows 100% discharge performance at -25C and >= 70% discharge performance at -20C; nothing about charge performance at temps other than 25C+/-2C.
So charge performance at temps lower than 25C is completely unspecified.
On top of that, if a cell fails the >=70% discharge at -20C, this could easily be the reason EVE sells these cheap cells off through grey market resellers.
So I’m happy with how my cells perform at 23-27C but will remain cautious about assuming that performance translates blindly down to -8C without verifying first.
Yes.
Yes, plated copper will be fine

As said, feel free to mix and match the advices. I was just describing what I think is best, but of course "best" depends on your specific needs and what you want...
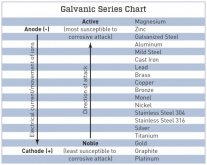
Basically the less dissimilar metals you have the better. For example if you start having zinc plated steel nuts, stainless studs, brass washers, copper busbars and aluminium terminals you'll have more problems no matter the environment...
So one last question then:
If I’ve got plated copper busbars on aluminum studs, would plated steel studs, nuts and washers count as adding another dissimilar metal or does the ‘plating against plating’ mean plated steel will behave about as well as plated copper (which I am not interested in).
Taking your words at face value, with aluminum terminals and plated copper busbars, II should either go with aluminum hardware or plated copper hardware (from which I prefer the idea of aluminum).
Plated steel would be easiest and cheapest if that will work about as well...
No problem, I can see you use your brain cells so you'll probably chose a good solution and don't have problems,
So don’t worry too much 
Yeah, right. Between clamping fixtures, avoiding corrosion, assuring grey-market cells are well-matched and properly top-balanced, selecting the correct BMS for your application, and then all the complexity of wiring and charging systems and voltages, if you are not comfortable ‘worrying’, this is probably not the right hobby for you ?