You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Why is bulk/absorption voltage used?
- Thread starter hwy17
- Start date
sunshine_eggo
Happy Breffast!
And in your opinion is a higher 1 voltage over 2 achieving a real benefit for LFP? A real difference in charging speed?
Yes. My 13.6V chart demonstrates that a lower absorption voltage took about 7.5 hours starting at 20A vs. a little over 5 hours.
In the NMC world are higher 1 voltages common as well?
No. There's effectively only a float voltage. NMC has a strong voltage to SoC correlation. It tends to stay very close to where you put it voltage-wise.
Every day I charge to 54.8V (3.92V/cell) and hold that voltage for as long as the sun can power the loads.
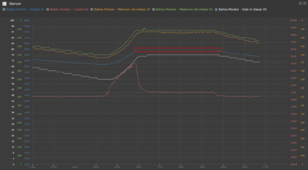
If my Batrium wasn't feeding the "charge" voltage to the system, I would simply set absorption to 54.8V for 1 hour and then set float just under that at 54.7V.
sunshine_eggo
Happy Breffast!
What happens if bulk, absorb, and float are all set to 54v?
Depends on the battery.
Say on the average Lifepo4 server rack batteryDepends on the battery.
sunshine_eggo
Happy Breffast!
What happens if bulk/absorb, and float are all set to 54v?
Edited. Bulk and absorption are the same voltage.
Say on the average Lifepo4server rack battery
Form factor doesn't matter.
You'd charge to a < 100% SoC slowly.
Some chargers like a lot of the older MPP Solar/Growatt don't function properly if bulk=float. They charge to absorption, hold awhile and then just stop charging.
Using the prior chart:
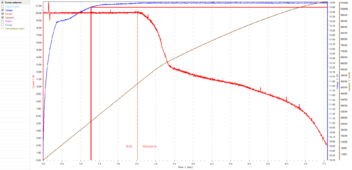
It would have started tapering current a full 1.25 hours earlier, and it would have terminated sooner. I just don't know what peak SoC will be at 3.375V or how long it will take to get there.
hwy17
Anti-Solar Enthusiast
I see one chart. Is there a different chart for a higher absorption voltage I'm missing?Yes. My 13.6V chart demonstrates that a lower absorption voltage took about 7.5 hours starting at 20A vs. a little over 5 hours.
sunshine_eggo
Happy Breffast!
I see one chart. Is there a different chart for a higher absorption voltage I'm missing?
No. I posted this as it was a surprise to me that I was able to get nearly to 100%. Past experience on single cell charging showed closer to 95%.
Here is a recent single cell charge I did on a 25.5Ah Topband 3C LFP cell:
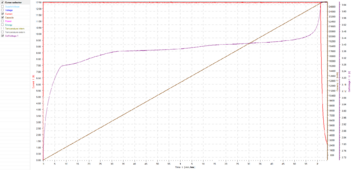
This is a 0.5C charge to 3.65V with a 0.05C tail current, so in a perfect world at peak current, it should take 2 hours, 2 minutes and 24 seconds to charge.
The charge time was 2 hours, 4 minutes and 22 seconds, so there was only about 2 minutes of absorption time, and it charged at peak current for almost all of the charge.
If I had only charged to 3.40V/cell:
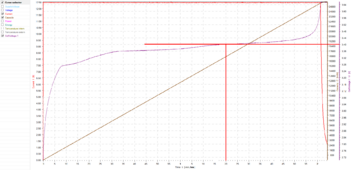
It would have started tapering current after only 1 hour and 20 minutes, and the charge would have taken notably longer.
Checkthisout
Solar Wizard
- Joined
- Nov 14, 2021
- Messages
- 4,856
You are just repeating the same point I am questioning that different voltage targets are necessary to speed up charging, and it doesn't look that true for Lifepo4.
There is only 1 voltage target during charging and that is the absorption voltage.
The battery will not reach full charge if it's only charged up to the float voltage.
The battery will be overcharged if left to float at a voltage that's any measurable voltage higher than it's rested fully charged voltage.
hwy17
Anti-Solar Enthusiast
@sunshine_eggo The 3.65 charge is very interesting to this discussion, thank you. And I appreciate the refutation of my hypothesis from someone who is familiar with another battery chemistry with one charging voltage. So I can be sure it's not that you're not getting my point.
I'm not quite understand your assumptive charting based on the crossing. In one example, the 3.65 chart, the original chart does go into CV right at the voltage crossing. I don't understand the mathematical mechanism, but it does. In the other chart though, the original chart does not go into CV at a crossing.
Also, these two tests are at different currents, which is going to affect the CC duration as well as the voltage. I'm not saying you said they were a 1 for 1, just thinking it through here.
I'm not quite understand your assumptive charting based on the crossing. In one example, the 3.65 chart, the original chart does go into CV right at the voltage crossing. I don't understand the mathematical mechanism, but it does. In the other chart though, the original chart does not go into CV at a crossing.
Also, these two tests are at different currents, which is going to affect the CC duration as well as the voltage. I'm not saying you said they were a 1 for 1, just thinking it through here.
Last edited:
hwy17
Anti-Solar Enthusiast
Don't get ahead of yourself here and weaken your argument.The battery will not reach full charge if it's only charged up to the float voltage.
Checkthisout
Solar Wizard
- Joined
- Nov 14, 2021
- Messages
- 4,856
Don't get ahead of yourself here and weaken your argument.
If the battery is dead, then you cannot reach the absorption voltage without exceeding the amperage limit.
If I took a lipo4 that is at 2.5 volts and applied enough juice to it to get the terminal voltage to 3.65 volts instantaneously, the battery would blow up and no else in town would have electricity to use while I charged it.
Checkthisout
Solar Wizard
- Joined
- Nov 14, 2021
- Messages
- 4,856
I'm not sure what your asking.
Lithium doesn't need a long absorption time so that step can be eliminated, in theory but balancing may not occur in some cases.
So all you need are bulk and float but cc and cv are still needed to protect the charger and battery.
Or are you arguing that we only need 1 voltage setting and that's "float"?
Lithium doesn't need a long absorption time so that step can be eliminated, in theory but balancing may not occur in some cases.
So all you need are bulk and float but cc and cv are still needed to protect the charger and battery.
Or are you arguing that we only need 1 voltage setting and that's "float"?
hwy17
Anti-Solar Enthusiast
I know, and you weren't too interested in thoughtfully exploring it. So I'm kind of done having to try to rephrase the question to you.I'm not sure what your asking.
Edit: Editing this now to say that was a bit too grumpy. I was just overreacting to having CC and CV and other "basic concepts" explained to me.
Last edited:
hwy17
Anti-Solar Enthusiast
I'm entertaining the idea that what if we did, would the difference in charge performance be as great as this community's consensus proposes.Or are you arguing that we only need 1 voltage setting and that's "float"?
The counter arguments are:
1. You wouldn't be in good balancing voltage.
2. The charge would be too slow.
And I'm saying would it really? I think you can balance at float and that is now more or less the consensus. And would it be that slow? If we're talking about 5% SOC left on the table, is that 5% worth packing these higher voltages in every day?
Checkthisout
Solar Wizard
- Joined
- Nov 14, 2021
- Messages
- 4,856
I'm entertaining the idea that what if we did, would the difference in charge performance be as great as this community's consensus proposes.
The counter arguments are:
1. You wouldn't be in good balancing voltage.
2. The charge would be too slow.
And I'm saying would it really? I think you can balance at float and that is now more or less the consensus. And would it be that slow? If we're talking about 5% SOC left on the table, is that 5% worth packing these higher voltages in every day?
1) What Voltage do you consider fully charged? Rested and while charging
2) What single voltage do you propose?
It's like filling a tire with air. If my compressor is at the same pressure I want my tire, then it fills a lot slower. If my compressor psi is well above that of the tire, it fills quickly.
If you're so overpaneled that you can charge at float voltage and make it through the night then you got it made.
hogback
New Member
I think the discussion here should be best taken to the optimal charging protocol for a single cell, not a series combination with a bms. If the 3.45 (or whatever) bulk limit, the 3.45 (or whatever) absorb, and 3.35 (or whatever) float is chosen because of balancing issues, so be it, but if it is because of electrochemical considerations of the electrodes, then that is more fundamental, and should be considered first.
I don't have a good answer, as I'm not knowledgeable with lifepos in particular, but I do understand electrodes, their thermodynamics, and kinetics pretty well, but more from a corrosion stance. I can imagine that modelling lifepo cathodes could be pretty complicated, as it involves solid state diffusion in the cathode. What I can say is that considering non-equilibrium kinetics (charging/discharging) is where a good deal of the complications arise. The most meaningful potential is that at open circuit, when there are no loads or charging, and after everything has relaxed, which can take some time. When you charge, there is an overpotential associated with ohmic loss in the electrolyte, an activation overpotential associated with driving the fundamental reaction a certain direction at a certain rate, and a diffusion overpotential, that results from the fact that some ions in solution have different diffusivities than others, and are essentially separated from one another (like a little capacitor) when they are pulled one way in an electric field, but at the same time are trying to even out concentrations due to diffusion. These overpotentials disappear when you stop charging and have no loads, after some time anyways. They are a strong function of current density, in varying and often complicated ways. What's determining the energy density of the system (roughly the soc) at this point is the electrochemical potential of the fundamental reactions, which is pretty straightforward, and the amount of 'stuff' at one electrode or the other.
All of the overpotentials result in efficiency losses, so you can see that the most efficient way to draw power from a battery, or charge it, is to draw infinitesimal currents, which is clearly not practical. It makes sense that you would try to hold a cell at its open circuit potential at full charge, which I understand is around 3.35V. It's entirely possible that some time is desirable at higher potentials for some reason related to the state of the electrodes - I just don't know.
I think what hwy17 is trying to get at is is there a point of ever being above 3.35 during a charge. The way I see it is yes, because for any meaningful charging current, there is an associated overpotential, so to snap back at the right open circuit potential of full-ish soc, you charge above it, but still below 3.65, and wait for current to taper down a bit. Outside of unknown (to me) electrode effects, you'd still get to the same soc by holding at 3.35 for infinite time, sort of like walking halfway to a wall each time you walk to it. The overpotentials diminish with decreasing current. So, in short, it is a 'time' reason. For a 16s battery, I can see there being reasons involved with the balancing dance, but these are separate.
I don't have a good answer, as I'm not knowledgeable with lifepos in particular, but I do understand electrodes, their thermodynamics, and kinetics pretty well, but more from a corrosion stance. I can imagine that modelling lifepo cathodes could be pretty complicated, as it involves solid state diffusion in the cathode. What I can say is that considering non-equilibrium kinetics (charging/discharging) is where a good deal of the complications arise. The most meaningful potential is that at open circuit, when there are no loads or charging, and after everything has relaxed, which can take some time. When you charge, there is an overpotential associated with ohmic loss in the electrolyte, an activation overpotential associated with driving the fundamental reaction a certain direction at a certain rate, and a diffusion overpotential, that results from the fact that some ions in solution have different diffusivities than others, and are essentially separated from one another (like a little capacitor) when they are pulled one way in an electric field, but at the same time are trying to even out concentrations due to diffusion. These overpotentials disappear when you stop charging and have no loads, after some time anyways. They are a strong function of current density, in varying and often complicated ways. What's determining the energy density of the system (roughly the soc) at this point is the electrochemical potential of the fundamental reactions, which is pretty straightforward, and the amount of 'stuff' at one electrode or the other.
All of the overpotentials result in efficiency losses, so you can see that the most efficient way to draw power from a battery, or charge it, is to draw infinitesimal currents, which is clearly not practical. It makes sense that you would try to hold a cell at its open circuit potential at full charge, which I understand is around 3.35V. It's entirely possible that some time is desirable at higher potentials for some reason related to the state of the electrodes - I just don't know.
I think what hwy17 is trying to get at is is there a point of ever being above 3.35 during a charge. The way I see it is yes, because for any meaningful charging current, there is an associated overpotential, so to snap back at the right open circuit potential of full-ish soc, you charge above it, but still below 3.65, and wait for current to taper down a bit. Outside of unknown (to me) electrode effects, you'd still get to the same soc by holding at 3.35 for infinite time, sort of like walking halfway to a wall each time you walk to it. The overpotentials diminish with decreasing current. So, in short, it is a 'time' reason. For a 16s battery, I can see there being reasons involved with the balancing dance, but these are separate.
TomC4306
Solar Obsessive
As previously stated, you won't fully charge the battery.What happens if bulk, absorb, and float are all set to 54v?
TomC4306
Solar Obsessive
I implore you to watch those two Andy at off-grid garage videos. Obviously you have not.@sunshine_eggo The 3.65 charge is very interesting to this discussion, thank you. And I appreciate the refutation of my hypothesis from someone who is familiar with another battery chemistry with one charging voltage. So I can be sure it's not that you're not getting my point.
I'm not quite understand your assumptive charting based on the crossing. In one example, the 3.65 chart, the original chart does go into CV right at the voltage crossing. I don't understand the mathematical mechanism, but it does. In the other chart though, the original chart does not go into CV at a crossing.
Also, these two tests are at different currents, which is going to affect the CC duration as well as the voltage. I'm not saying you said they were a 1 for 1, just thinking it through here.
TomC4306
Solar Obsessive
Right but if you zoom in on sunshines graph, you will notice that at 13.6 volts it required over 5 hours of absorption to get full. So first it required time to charge to the voltage and then it required five more hours to fully saturate the cells at that voltage 13.6. the sun isn't up for that long. Most people here are cycling their batteries everyday so all of this charging and absorbing and floating has to happen within the context of one sunny day.I think the discussion here should be best taken to the optimal charging protocol for a single cell, not a series combination with a bms. If the 3.45 (or whatever) bulk limit, the 3.45 (or whatever) absorb, and 3.35 (or whatever) float is chosen because of balancing issues, so be it, but if it is because of electrochemical considerations of the electrodes, then that is more fundamental, and should be considered first.
I don't have a good answer, as I'm not knowledgeable with lifepos in particular, but I do understand electrodes, their thermodynamics, and kinetics pretty well, but more from a corrosion stance. I can imagine that modelling lifepo cathodes could be pretty complicated, as it involves solid state diffusion in the cathode. What I can say is that considering non-equilibrium kinetics (charging/discharging) is where a good deal of the complications arise. The most meaningful potential is that at open circuit, when there are no loads or charging, and after everything has relaxed, which can take some time. When you charge, there is an overpotential associated with ohmic loss in the electrolyte, an activation overpotential associated with driving the fundamental reaction a certain direction at a certain rate, and a diffusion overpotential, that results from the fact that some ions in solution have different diffusivities than others, and are essentially separated from one another (like a little capacitor) when they are pulled one way in an electric field, but at the same time are trying to even out concentrations due to diffusion. These overpotentials disappear when you stop charging and have no loads, after some time anyways. They are a strong function of current density, in varying and often complicated ways. What's determining the energy density of the system (roughly the soc) at this point is the electrochemical potential of the fundamental reactions, which is pretty straightforward, and the amount of 'stuff' at one electrode or the other.
All of the overpotentials result in efficiency losses, so you can see that the most efficient way to draw power from a battery, or charge it, is to draw infinitesimal currents, which is clearly not practical. It makes sense that you would try to hold a cell at its open circuit potential at full charge, which I understand is around 3.35V. It's entirely possible that some time is desirable at higher potentials for some reason related to the state of the electrodes - I just don't know.
I think what hwy17 is trying to get at is is there a point of ever being above 3.35 during a charge. The way I see it is yes, because for any meaningful charging current, there is an associated overpotential, so to snap back at the right open circuit potential of full-ish soc, you charge above it, but still below 3.65, and wait for current to taper down a bit. Outside of unknown (to me) electrode effects, you'd still get to the same soc by holding at 3.35 for infinite time, sort of like walking halfway to a wall each time you walk to it. The overpotentials diminish with decreasing current. So, in short, it is a 'time' reason. For a 16s battery, I can see there being reasons involved with the balancing dance, but these are separate.
So, yeah, charging speed is important to this endeavor.
TomC4306
Solar Obsessive
Go watch those videos.I'm entertaining the idea that what if we did, would the difference in charge performance be as great as this community's consensus proposes.
The counter arguments are:
1. You wouldn't be in good balancing voltage.
2. The charge would be too slow.
And I'm saying would it really? I think you can balance at float and that is now more or less the consensus. And would it be that slow? If we're talking about 5% SOC left on the table, is that 5% worth packing these higher voltages in every day?
This betrays your ignorance of the chemistry.
Cell imbalance only exposes itself in the upper part of the knee of the charge curve.
Charge curve for this chemistry at the margins is logarithmic....
In a logarithmic area is where you do cell balancing, not in the fat flat center of the curve.
Similar threads
- Replies
- 13
- Views
- 438
- Replies
- 9
- Views
- 323
- Replies
- 57
- Views
- 2K
- Replies
- 3
- Views
- 236


