hankcurt
Solar Enthusiast
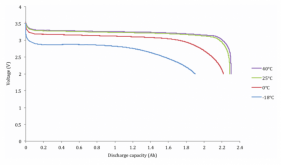
Internal resistance of a battery becomes easier to comprehend when you look at the processes in the battery in mechanical terms. This is a valid way to look at it because in the charging and discharging process, you are physically relocating ions from one location in the battery to another location. The ions have to make their way out of a graphite solid, travel though a separator material, and then travel into another solid made out of iron and phosphate atoms. As stuff moves past other stuff, it bumps around and loses some energy as heat, so the materials of construction for the battery are selected to make the pathways as open as possible.
However, if manufacturing quality is poor, these pathways might not be optimally placed. Or if a battery is abused, the pathways might get damaged, or plugged by molecules that were created in side reactions from over charging. The materials the pathways are made out of can get physically cracked and degraded through normal use. This is why measuring internal resistance can tell you if one battery cell is in similar condition to another battery cell. But you have to do the measurement under the same conditions because the state of charge (how many lithium ions are already parked in the pathways), temperature, and rate of charge or discharge (how many lithium ions you are trying to move at once) all affect the reading.
I found this animation to show lithium ions moving in the anode material. Before you run it, grab the image and rotate it around, and from one direction you will see that it has large openings. These are the pathways the lithium uses. Thought this might help some people who are visual learners.
However, if manufacturing quality is poor, these pathways might not be optimally placed. Or if a battery is abused, the pathways might get damaged, or plugged by molecules that were created in side reactions from over charging. The materials the pathways are made out of can get physically cracked and degraded through normal use. This is why measuring internal resistance can tell you if one battery cell is in similar condition to another battery cell. But you have to do the measurement under the same conditions because the state of charge (how many lithium ions are already parked in the pathways), temperature, and rate of charge or discharge (how many lithium ions you are trying to move at once) all affect the reading.
I found this animation to show lithium ions moving in the anode material. Before you run it, grab the image and rotate it around, and from one direction you will see that it has large openings. These are the pathways the lithium uses. Thought this might help some people who are visual learners.