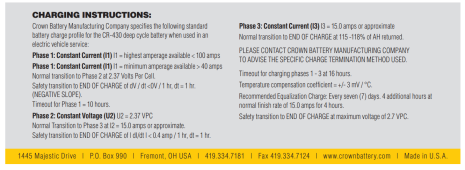
The tech at rolls specifically states to use low current which is opposite to what you've insisted. The manual doesn't specify equalisation current but it does specify 15.6 to 15.9 voltage, lower than the attempted repair charge. Not sure why you're getting so worked up about it.
LOL... not worked up. You'll just understand it all better once you actually READ the manual. You're looking for solutions to a problem you can't quantify or understand without gathering data first.
Since you seem to think this is meaningful:
"In both cases, the fact that the battery "won't take a charge" is a result of improper charging procedures which allowed the sulfate to harden. In most instances, it is possible to salvage a battery with hardened sulfate. The battery should be charged from an outside source at 2.6 to 2.7 - volts per cell and a low current rate (approximately 5 Amps for small batteries and 10-Amps for larger ones) until the specific gravity of the electrolyte starts to rise."
Let's look at the words and their meaning:
CHARGED
AT (not below) 2.6-2.7V (funny how the manual specified EQ voltage is 2.65V/cell)/CELL)
You understand that voltage responds to current? If you apply 5-10A on a battery, its voltage will raise in accordance with its resistance and state of charge. If it fails to achieve 2.6-2.7V, you're not getting there, so you need to increase current.
You're thinking "low current" is the key, but it's "low current
AT 2.6-2.7V."
Now what do you do? It's actually ambiguous. Once SG starts to rise, what now? It doesn't say. Do you let the voltage fall and just keep applying 5-10A? NO!!!!! NOT ON YOUR LIFE!!!! The MANUAL says that's counter productive as charge rates below 0.1C may increase the tendency for sulfation to form during charge (it even recommends 10% minimum in your link).
I'll line it out for you so you don't have to read it:
Check fluid levels. Fill only with distilled water such that all plates are covered. Do not top off cells.
Charge battery @ 0.1-0.2C (71-142A) to 7.5V.
Absorption charge at 7.5V for:
T = .042 * 710 / I where T = hours of charging and I = bulk current
Alternatively, until current drops to 0.02C (14.2A) @ 7.5V.
Once charge is completed, float for two hours at 6.75V (this allows SG to stabilize).
Check SG by drawing and expelling electrolyte 3 times, draw in a 4th time and take reading (this ensures you don't get stratified surface reading).
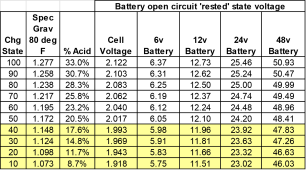
If SG below 1.265, EQ.
EQ:
Charge at 0.05-0.1C (35.5-71A) until 7.95V.
Hold 7.95V.
Periodically monitor SG.
Terminate EQ:
1) when battery temp exceeds 125°F (allow battery to cool and restart EQ if SG is still rising)
2) when SG stops increasing.
3) when SG > 1.265
Batteries that don't respond to the above are done for. You may need to conduct the process described in the link, but what you will find is that when the battery starts to "wake up" and SG increases, the current required to maintain the 7.95V begins to increase markedly above 5-10A. At that point, you should transition to a normal charge, or you will actually damage the battery.