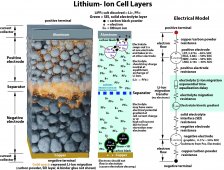
The attached picture shows two 120 AH LiFePO4 cells that have been overcharged. Stupidly, I bypassed my controller, and connected a trickle charger directly, and then promptly forgot about it. I found them several days later swollen as shown in the picture with well over 7 volts (2 cells) . I have been using them since then, and they seem to be working fine, although I haven't done a load test on them. The sides are still firm, and when I squeeze them with my hand, I cannot compress them at all; I haven't tried any tools. They have not leaked, and there is no evidence that the seals on top have released any gas.
Please help me with your experience.
Q: Can I continue to use them? Is there any risk? I would like to do a load test and continue to use them.
Q: What is going on inside? Is the LiFePO4 cell expanded, or only the case?
Thanks.
Doug
I hesitate to mention this, but every time I post something on a forum nowadays, I get an answer from someone who has the most posts on the forum, but is not particularly knowledgable. Thankfully, I have been blessed with a good BS detector. 'Nuf said.
View attachment 113654