I would like to challenge anyone reading this to find a photo or video of a LiFePO4 creating internal combustion from thermal runaway event.
I keep seeing people comment that LiFePO4 can combust, but UL listed companies and battery studies are telling me otherwise. Where are people coming up with this idea? LiFePO4 raw cells are being constructed in packs without individual cell fusing, and no one is blinking an eye.
But there are articles from reputable people (Jack Rickards videos as well) stating that they can combust.
So if someone can PLEASE email me evidence of LiFePO4 combusting from thermal runaway, I would love to see it (my email is willprowseiv@gmail.com)
Here is some of my proof that LiFePO4 is NOT combustible:
https://www.osti.gov/servlets/purl/1395736
https://relionbattery.com/uploads/images/misc/Relion_SafetyLithiumBattery.pdf
https://www.mdpi.com/1996-1073/11/9/2191/pdf
So the cobalt based ones have oxygen loosely attached, and when hot, can cause combustion. This makes it more hot, and thermal runaway goes nuts! And LiFePO4 does not have this problem, no cobalt. Am I missing something?
And this is what combustion is: high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion in a fire produces a flame, and the heat produced can make combustion self-sustaining. LiFePO4 heat generation, is not self-sustaining, and does not create enough heat to combust cells next to it.
It will create heat, but not much. There is no "cascade" effect, from what I can tell. It is not considered a oxide based lithium ion chemistry. And I remember when learning about rocket fuels (scott manley's youtube channel!), that pressure and temperature have a huge part to play in this reaction.
But what I find interesting is the thermal runaway properties of LiFePO4 at various temperatures... it does not increase.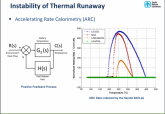
And simpli-phi, which creates high quality, UL listed battery packs, states this as well:

Technically, any battery can pose a fire threat because it can cause a conductor in a system to heat up (if OCPD not present), and set something else on fire. But the internal chemistry of a lifepo4, as I can tell and what safety studies have shown, is that it cannot. If you disagree with this, please let me know below.
I keep seeing people comment that LiFePO4 can combust, but UL listed companies and battery studies are telling me otherwise. Where are people coming up with this idea? LiFePO4 raw cells are being constructed in packs without individual cell fusing, and no one is blinking an eye.
But there are articles from reputable people (Jack Rickards videos as well) stating that they can combust.
So if someone can PLEASE email me evidence of LiFePO4 combusting from thermal runaway, I would love to see it (my email is willprowseiv@gmail.com)
Here is some of my proof that LiFePO4 is NOT combustible:
https://www.osti.gov/servlets/purl/1395736
https://relionbattery.com/uploads/images/misc/Relion_SafetyLithiumBattery.pdf
https://www.mdpi.com/1996-1073/11/9/2191/pdf
So the cobalt based ones have oxygen loosely attached, and when hot, can cause combustion. This makes it more hot, and thermal runaway goes nuts! And LiFePO4 does not have this problem, no cobalt. Am I missing something?
And this is what combustion is: high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion in a fire produces a flame, and the heat produced can make combustion self-sustaining. LiFePO4 heat generation, is not self-sustaining, and does not create enough heat to combust cells next to it.
It will create heat, but not much. There is no "cascade" effect, from what I can tell. It is not considered a oxide based lithium ion chemistry. And I remember when learning about rocket fuels (scott manley's youtube channel!), that pressure and temperature have a huge part to play in this reaction.
But what I find interesting is the thermal runaway properties of LiFePO4 at various temperatures... it does not increase.

And simpli-phi, which creates high quality, UL listed battery packs, states this as well:
Technically, any battery can pose a fire threat because it can cause a conductor in a system to heat up (if OCPD not present), and set something else on fire. But the internal chemistry of a lifepo4, as I can tell and what safety studies have shown, is that it cannot. If you disagree with this, please let me know below.





