You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Research on cell compression / bracing
- Thread starter gonmag8
- Start date
I want some of those cells. Cycled 13000 times - discharging at 2C to 2.7V and charging at 1C to 4.15V
I didn’t see what type of chemistry they were - i doubt it’s LiFePO4.
Quote: "Based on the ICP-OES results, the composition of the cathode active material of Cell 1 (reference cell) is estimated as Li1.0 Ni0.4 Co0.3 Mn0.3 O2"
So, NMC cells.
hankcurt
Solar Enthusiast
Even though the battery being tested in this study is a different lithium ion chemistry than the lithium iron phosphate most of us are using, there are still some good concepts to take away from the paper.
First, the internal structure of the battery is put under mechanical stress as the battery state of charge changes and ions migrate back and forth between the anode and cathode. In their conclusion, they attributed much of the physical swelling of the prismatic cell to cracking, electrode de-lamination, and lithium plating. They noted that the plating problem in the braced cell was greatly reduced because the layers inside the battery were less prone to separate, and this led to a reduction in swelling and a greater retention of charge capacity.
Unfortunately, their cell brace did not allow for measurement of the pressures applied to the cell. It does appear that it is an inflexible brace, so the pressure probably changed with state of charge and age. Interestingly, they say that both the braced and unbraced cell cathodes suffered from cracking. However, it was the delamination and lithium plating that caused most of the capacity loss, and these factors were improved by bracing.
So essentially, by building a battery cell fixture that applies pressure to the cells within the limits of the manufacturers specifications, you are keeping the materials inside the battery tightly connected as the individual layers expand and contract. By doing that, you are preserving contact for ion exchange across the layers and preventing lithium from plating the surface of the materials, and that keeps the lithium in circulation in the battery and helps preserve its capacity.
Its nice to have some experimental data to support the practice of putting cells under some pressure as they are operating.
Good find.
First, the internal structure of the battery is put under mechanical stress as the battery state of charge changes and ions migrate back and forth between the anode and cathode. In their conclusion, they attributed much of the physical swelling of the prismatic cell to cracking, electrode de-lamination, and lithium plating. They noted that the plating problem in the braced cell was greatly reduced because the layers inside the battery were less prone to separate, and this led to a reduction in swelling and a greater retention of charge capacity.
Unfortunately, their cell brace did not allow for measurement of the pressures applied to the cell. It does appear that it is an inflexible brace, so the pressure probably changed with state of charge and age. Interestingly, they say that both the braced and unbraced cell cathodes suffered from cracking. However, it was the delamination and lithium plating that caused most of the capacity loss, and these factors were improved by bracing.
So essentially, by building a battery cell fixture that applies pressure to the cells within the limits of the manufacturers specifications, you are keeping the materials inside the battery tightly connected as the individual layers expand and contract. By doing that, you are preserving contact for ion exchange across the layers and preventing lithium from plating the surface of the materials, and that keeps the lithium in circulation in the battery and helps preserve its capacity.
Its nice to have some experimental data to support the practice of putting cells under some pressure as they are operating.
Good find.
So essentially, by building a battery cell fixture that applies pressure to the cells within the limits of the manufacturers specifications, you are keeping the materials inside the battery tightly connected as the individual layers expand and contract. By doing that, you are preserving contact for ion exchange across the layers and preventing lithium from plating the surface of the materials, and that keeps the lithium in circulation in the battery and helps preserve its capacity.
Its nice to have some experimental data to support the practice of putting cells under some pressure as they are operating.
This is not the first time research has shown this. I'll try to remember some other papers...
hankcurt
Solar Enthusiast
I don't think this is true in respect to prismatic cells, and not all cells have the same recommendation.It has always been recommended to restrain cells.
Even within the same EVE 280ah product line, the spec sheet recommendations have changed, either due to experience gained as cells were in service, or due to changes in cell manufacture. I think the research above is interesting in the regard that it provides a window into what the company may have learned that caused them to update their spec sheet.
As an example, I've attached version B of the EVE 280ah spec sheet from 2018. It makes no mention of 'fixture' or 'compression'. The only structural recommendation for the cell holder is that it protect the cells from impact damage.
(Note: The attached spec sheet is old. Newer spec sheets from the 'Resources' page should be used for current information)
Attachments
Last edited:
RCinFLA
Solar Wizard
- Joined
- Jun 21, 2020
- Messages
- 3,565
This paper is on non-LFP cells. Cathodes in non-LFP cells are usually the weak link in Li-Ion battery longevity.
LFP cathodes are much more rugged due to their inter-lattice support structure that does not exist on other Li-ion chemistry cathodes. Non-LFP cathode lattice, when discharged, is like a multi-layer parking garage with limited vertical support pillars. Downside of LFP is their lower energy density due to lower cathode polarization voltage. (3.2v vs 3.8v cells)
The lithium plating on anode side stated in paper is likely due to cathode degradation on non-LFP cells that results in limited recharging to cathode lattice locations due to positive electrode cathode damage. With limited cathode lattice places for lithium ions to go during charging they do damaging recombination creating lithium plating. Lithium plating most often occurs when lithium ions hang around too long in graphite electrode, outside their safe graphite lattice parking spots, allowing escaping electrons from anode electrode to combine and create lithium metals that create metal dendrites.
This is what happens on LFP cells when attempting charging at cold temps when the lithium-ion migration rate becomes very sluggish giving more time for electrons in anode electrode to combine with lithium ions attempting to exit graphite anode to get back to cathode side. The SEI layer coating grown around graphite granules is there as a barrier to keep electrons within higher conducting graphite but some do jump the SEI barrier and do damage in electrolyte by creating undesired parasitic chemical compounds.
The primary benefit of cell compression is reduction in possible graphite delamination from copper foil. Graphite expands about 9-11% in volume when fully charged, same for all Li-ion chemistries. Compression on cell of non-LFP cathodes is more likely to cause cathode lattice damage when it is discharged in a structurally weaker condition.
LFP cathodes are much more rugged due to their inter-lattice support structure that does not exist on other Li-ion chemistry cathodes. Non-LFP cathode lattice, when discharged, is like a multi-layer parking garage with limited vertical support pillars. Downside of LFP is their lower energy density due to lower cathode polarization voltage. (3.2v vs 3.8v cells)
The lithium plating on anode side stated in paper is likely due to cathode degradation on non-LFP cells that results in limited recharging to cathode lattice locations due to positive electrode cathode damage. With limited cathode lattice places for lithium ions to go during charging they do damaging recombination creating lithium plating. Lithium plating most often occurs when lithium ions hang around too long in graphite electrode, outside their safe graphite lattice parking spots, allowing escaping electrons from anode electrode to combine and create lithium metals that create metal dendrites.
This is what happens on LFP cells when attempting charging at cold temps when the lithium-ion migration rate becomes very sluggish giving more time for electrons in anode electrode to combine with lithium ions attempting to exit graphite anode to get back to cathode side. The SEI layer coating grown around graphite granules is there as a barrier to keep electrons within higher conducting graphite but some do jump the SEI barrier and do damage in electrolyte by creating undesired parasitic chemical compounds.
The primary benefit of cell compression is reduction in possible graphite delamination from copper foil. Graphite expands about 9-11% in volume when fully charged, same for all Li-ion chemistries. Compression on cell of non-LFP cathodes is more likely to cause cathode lattice damage when it is discharged in a structurally weaker condition.
Jimmyburnworld
New Member
- Joined
- Aug 2, 2022
- Messages
- 13
I'm just in the process of sorting my cell compression for my EVE 280s so this thread is well timed and interesting food for thought.
I've put the following together in CAD, the idea being that the bracing is assembled with 4 lengths of threaded rod. The steel angle is 25 x 3mm and the blocks are 3D printed plastic. Specifically I'd welcome thoughts on the single block in the middle. My thinking is that this is where most of the distortion of the cell occurs so this is the key point to apply pressure.
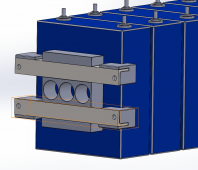
I would then use self adhesive foam tape between cells and repeat the bracing at the other end of the pack.
I've put the following together in CAD, the idea being that the bracing is assembled with 4 lengths of threaded rod. The steel angle is 25 x 3mm and the blocks are 3D printed plastic. Specifically I'd welcome thoughts on the single block in the middle. My thinking is that this is where most of the distortion of the cell occurs so this is the key point to apply pressure.

I would then use self adhesive foam tape between cells and repeat the bracing at the other end of the pack.
Similar threads
- Replies
- 32
- Views
- 1K
- Replies
- 1
- Views
- 173
- Replies
- 9
- Views
- 602


