Storing thermal energy in an insulated box containing sand is, for me, a bad idea for those reasons :
1 - Specific heat capacity of water (4000 J/K/kg) is 4.5 times the sand one (850 J/K/kg) => Even if you heat sand at a higher temperature there is still a big margin. Sand weight something between 1500 kg (dry) and 2000 kg (wet) per cubic meter, for a same volume of material (water/sand) you get a 1.5x to 2x factor in favor of sand. That still, for a same volume, a factor of (4000/850)/1.5 = 3.1x (dry sand) and 2.3x (wet sand)
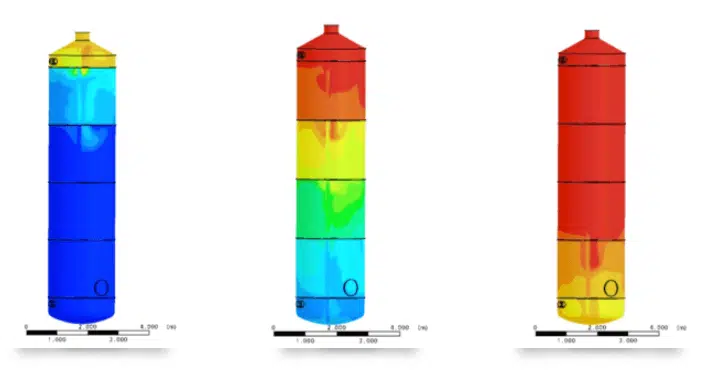
2 - Water like all fluids, is subject to convection currents. In a vertical tube, hot water rise and cold water sink In a vertical tank full of calm water there a gradient of temperatures, hot at the top, cold at the bottom => you can have water at 90°C at the top and 10°C at the bottom AND even after hours, it will stay a gradient.
The advantage of this... you can inject thermal energy in one point and it will "stratify", it's easy to know when youir battery is empty or full : Empty = Low temperature at the top, Full = high temperature at the bottom.
3 - It kind meet the first reason, sand will need to be heated at high temperature, the delta between the inside temperature and the outside one will be pretty high and this will imply higher loses.
4 - Even if the op build a sand storage... he will still need water or air tubes to convey the heat somewhere usefull.
I will give my case, cause i though of it before building my house, i got a 1000l thermal tank, it contain something like 40kWh of energy when full, i can take a shower or heat my house with the energy stored in it. I can heat it with PV panels or with my wood heater. It's very flexible !
You are ignoring that sand-based heat storages can store many times the amount of energy that can be stored in a water tank of a similar size or, put another way, you need 2000x the mass of water to store the same amount of energy as you can at moderate (1/3 the maximum pre-phase-change-temperature) in sand.
The whole ethos behind a sand battery is as a high-temperature and high-capacity energy store. If you don't want or need high temperature or high capacity, don't use it.
But, for high energy storage in a small volume at a minimal cost, water can't compete.
The cost disparity between a vessel that can store an equivalent amount of kJ of energy in water and sand is high.
Then you've got insulation costs, piping costs, etc. which purely from the size of the water reservoir vs that of sand is many times greater.
Focusing on the specific energy storage only, overlooks the key benefits of sand as an energy storage medium - that being price, compactness, ease of application.
Water has a specific heat of 4.19 kJ/LoC
Sand has a specific heat of 1.336 kJ/LoC (using L rather than kG as size is important - just ask my wife!)
At 600C 1L of sand has 801kJ of stored energy and can hold 1000C more -or 2137.6kJ of stored energy before the silica sand fuses.
At 99C (the highest you can go with water without a phase change) 1L of water has 414kJ of stored energy, and can't go any higher at ambient pressure.
So, to store the same amount of energy as 200L/320kg of sand at 600C - 160MJ - you would need 386,490L or 38CuM of water at 99C - 2000 x the volume.
(its 11pm - my math could be flaky)
Something else I haven't worked out is how you get the energy out of water.
Say you have 500W of solar heating a 2000W element in 1 CuM of water. You start out at ambient temperature of 5C and want to warm a house. Warming the house means getting a room to, say, in excess of 18C you need to store 54,470kJ
You won't have enough hours in a day to raise 1000L of water to 18C using a 500W panel - which can create around 2kW per day of sunshine or 7200kJ
54,470kJ / 7200kJ = it will take 7.5 days, assuming negligible losses due to extremely efficient insulation
After you have reached 18C in your 7.5 days of generation, what happens when you tap that heat? If you pull more than 7200kJ, you are taking more than you are putting in and going backwards.
So you need more energy generation.
The very thing that you posited as a positive ends up, compared to sand, to be a negative - convection means you need to heat the entire mass of water to get any benefit of the energy stored, where the carrying capacity of sand is such that what you store is very hot near the element, dropping inversely squared the further you go from the source of energy. Unlike water, you can get 18C out of the sand close to the element much easier and if you don't use it all, the heat keeps building and being stored at 100% efficiency. It's only when the entire mass of sand becomes saturated with energy are you going to see losses due to imperfect insulation.
To conclude - you describe things in terms of 'good' and 'bad', where I look at them in terms of overall effectiveness for the task required.
in the case of heating air to warm a dwelling, sand has an unparalleled price/performance ratio and a huge capacity advantage over water.