I mentioned on Tuesday that my entry's would slow down a bit while we wait for updates from Daniel - well, that changed yesterday. Since I am not properly trained nor have a degree in chemistry (besides high school lvl), for me to find solutions that might be beneficial are slim, but I did mention that I want to contribute as much as possible and I might just have something. I say might because this could be a dud for reasons beyond me.
I sat down and did a search for "diy Nafion" on YouTube and indeed, a video popped up [How to make alkaline membrane for fuel cell] and that seemed relatively straight forward and I was going to mention this video and now I have... LOL. I figured I would check the channel for other interesting stuff and indeed, there was one. [Battery using ion conducting ceramic separator and dual electrolyte]. If you scroll down and read in the comments section, he does mentions - translated from Portuguese - in quote since it is only supporting info:
Ceramic ion filter, interesting. So I did a search for ceramic water filter and many hits appeared. I expanded the search to "ceramic ion exchange membrane" and after scrolling a bit .. ding ding ding. Something of huge interest popped up and my gut feeling told me this could be it. This could be the membrane this cell needs in order to be even more cost effecting than using CMI-7000 and the overall cost of that membrane was to be honest, one of the reasons I wanted to explore some options. I have incorporated the interesting bits from the article:
Catalyzing Commercialization: Flexible Ceramic Membranes Enable Low-Cost Energy Storage - Feb 2019
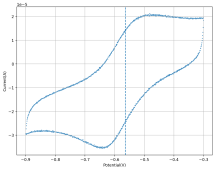
Molecular separations are at the heart of chemical engineering, and nanostructured membranes are emerging as a low-cost alternative to traditional separation processes. However, most membranes struggle to maintain performance in harsh environments. For example, redox flow batteries (RFBs) rely on a cation exchange membrane to store energy. To create an RFB capable of storing enough energy to power a city (>1 MWh) for 20+ years without capacity loss, an extremely robust membrane chemistry would be required, such as a perfluoro sulfonic acid (PFSA) membrane (NAFION etc). However, PFSA is expensive to manufacture, has mechanical and performance issues, and generates substantial quantities of bio accumulative and toxic substances during production. In many cases, PFSA membranes account for more than 20% of the total battery system costs. Researchers have been searching for more than three decades to find a material capable of delivering better performance at a lower cost.
With funding from the National Science Foundation (NSF), researchers at Membrion, Inc., have patented a potential solution that uses the desiccant commonly found at the bottom of a beef jerky package. Membrion has developed a self-assembly process for silica gel that enables its use as a low-cost and high-performance ceramic cation-exchange membrane.
“We were trying to lower the costs of RFBs by finding a cheaper alternative to PFSA that could handle the extreme conditions. Ceramics seemed like a logical choice, but they tend to be brittle and not well suited for the high compression in stack applications like RFBs. Our real innovation was in making a flexible and compressible ceramic membrane,” says Membrion’s Founder and Chief Technology Officer, Greg Newbloom.
This novel membrane processing method starts with a
nonwoven glass mesh commonly used as roofing insulation in the construction industry. Membrion scientists add a polymeric edging to the outer rim of the mesh to act as a gasket for the compressive loads in stack environments. They dip this polymer-edged glass mesh in a silica solution that wicks into the mesh, and then dip the mesh in a commodity acid. The end product is a percolated nanoporous ceramic membrane with a 2-in. bending radius.
“The acid dip is where the real science happens,” Newbloom says. “The acid causes the silica to electrostatically destabilize and form a network of nanopores. By controlling the kinetics of the process, we can make pores of different sizes and network structure. We use size and charge screening to dictate which molecules pass through the membrane and which are rejected.”
Membrion, Inc have two patents which is linked to under sources.
1. WO2020247467A1 CERAMIC CATION EXCHANGE MATERIALS
2. WO2020247472A1 CERAMIC ANION EXCHANGE MATERIALS
Closing words.
If this membrane turns out to be a viable option, then it would be advantageous to incorporate a diy process just like Daniel was working on regarding the cellulosa+PVA. Now, I don't know how much our chemists know, so I dug up an article / book called:
Current Trends and Future Developments on (Bio-) Membranes - Silica Membranes: Preparation, Modelling, Application, and Commercialization - Chapter 1 is literally called: Chapter 1 - Preparation of Silica Membranes by Sol-Gel Method - the book is linked down bellow. I will take a look what it has to offer.
I sincerely hope this membrane is the solution because if so, then this battery will be made using 3 of the most abundant elements: Silicon, Iron and Manganese - wouldn't that be awesome
... waiting for feedback
Sources:
-

www.aiche.org
-
Membrion's unique Electro-Ceramic Desalination technology can remove pollutants better, with less overhead than traditional wastewater treatment solutions.
membrion.com
-
Espacenet: free access to millions of patent documents. Find out if your invention is unique or if other inventors have filed patent applications that are considered to be prior art.

worldwide.espacenet.com
-