What color nail polish works best? Wait, that wasn't my question....I have done some first tests using the following architecture:
View attachment 171505
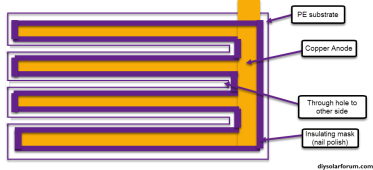
Basically strips of copper tape are first placed on a square of PE sheet, then a copper tape disc (sanded to a clean surface) and grafoil strip are placed on the anode and cathode sides respectively. A carbon felt is then glued to the grafoil's corners to secure it in place, the copper tape disc adheres using its adhesive. All exposed conductor surfaces are then insulated using nail polish to prevent any electrochemical behavior except for on the carbon felt and the copper disc anode. I used a 20m ZnCl2 + 5m NH4I solution (I ran out of potassium iodide, so I used ammonium iodide for the test). This is a Water-in-salt electrolyte (WISE). The setup is fully immersed in around 2mL of the solution.
As you can see, the above geometry ensures the Anode doesn't face the cathode and there are no direct paths for dendrites to cause a short. They would need to grow all around the electrolyte to achieve that.
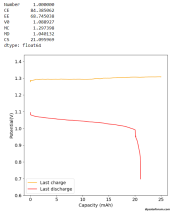
I did a couple of cycles at a current density of around 2mA/cm2, charging to 0.5mAh and discharging to 0.7V. The results are promising:
View attachment 171521
The Coulomb efficiency is quite high, above 90%, so we definitely do not get any major soluble triiodide formation in the electrolyte. All the iodide seems to plate as elemental iodide in the carbon felt (in agreement with published WISE results using KI+ZnCl2).
The loss in energy efficiency from the distance between the electrodes also seems to be limited, thanks to the high conductivity of the WISE electrolyte, even though the distance from anode to cathode is quite large as you need to go all around the PE sheet. Plating on the copper electrode looks very regular, at least from a first glance. There aren't any discernable dendrites at this state of charge (SOC). Note that higher EE value are likely achievable when charging at lower current density.
Note that the theoretical energy density you could get from this electrolyte is around 134 Ah/L (around 160 Wh/L at 1.2V) . For reference lithium iron phosphate is normally at around 220 Wh/L. The volume of the cathode material used is 0.26mL, so I would expect the cathode to hold 35mAh at a max.
A battery with this type of backplated configuration cannot be easily rolled, so likely instead of going for very fine separators and high voltage efficiencies a battery like this would work more like a lead acid battery, with larger cells stacked in rows. Basically arrays like the one I showed above stacked together to fill a box with the electrolyte then poured over to fill all the space and fill all the felt (which might require some vacuum to fully achieve wetting of the entire all the surface area.
I am now cycling this battery to 25mAh, which will take a long time per cycle, but I just want to see if it reaches the capacity and how dendrites behave at high SOC.
Are both the grafoil and the carbon felt part of the cathode? Would it work the same with just one or the other?