I received my new carbon paper electrode materials so I decided to continue my tests with them.
I started testing the P50 carbon material, which is working fine.
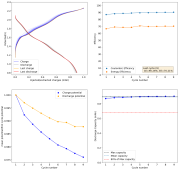
I wanted to go to higher capacity values with smaller separator length, but noticed I was getting a lot of dendrites really quickly with the MnCl2+ZnCl2 solutions, without being able to get any higher capacity with high coulombic efficiency. When using these solutions, all I could get was around 0.5mAh, if I tried charging any higher, the CE would drop dramatically and dendrites would form very quickly. I didn't add any of these tests to the experiment log as I rarely got more than 5 cycles at these capacities.
Since charging to higher capacities involved going to voltages above the 2V mark for a significant period of time, I believe the higher charging generated significant amounts of Cl2, which caused very high losses of CE.
For this reason, I decided to go back to MnSO4 + ZnSO4 electrolytes. I prepared a 1.5m MnSO4 + 1.5m ZnSO4 electrolyte, saturated with K2SO4 using 15% vinegar/80% distilled water as the solvent. I then ran tests using 3 fiberglass separator layers, a Zn anode and a P50 carbon cathode. All charging and discharging being done at 5mA/cm2.
I noticed something interesting. When you start charging these batteries, you initially get to very high voltages quite quickly (getting above 2.2V before getting to 0.5mAh) but as you go to higher voltages your charging voltage drops on each cycle. If you for example charge to 1mAh and cycle there a few times, but then only charge/discharge the battery to a lower threshold, say 0.5mAh, you get much better CE values.
This is because there are mainly two sets of reactions that happen in this battery. Initially you have Mn2+ ions in solution and these are oxidized upon charging to form MnO2 in the cathode. When you have MnO2 formed, you can then store H+ and Zn2+ ions inside the MnO2 structure - provided it's the correct type of MnO2 - but only when you already have formed the MnO2 in the cathode. This is why many papers, especially those using sulfates, will have some sort of preconditioning process before the batteries are cycled.
These are the half reactions that happen upon initial charging of the battery:
Mn2+ + 2H2O ⇌ MnO2(s) + 4 H+ + 2 e− -1.224V
Zn2++ 2 e− ⇌ Zn(s) -0.7618V
These can only happen at around 1.98V, which is the thermodynamic potential of the full reaction at 25C. Most likely there is some over potential due to internal resistance, reason why we only reach the charging plateau of this reaction at around 2.1-2.2V. At least in the batteries you've seen I've built.
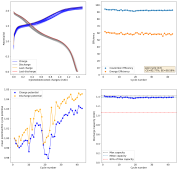
In the 1K cycle experiment we saw how initially the potential was very high and with time it decreased to where the average charging potential was much lower than the >1.98V needed to create MnO2. This is because, with time, we shift from a reaction where we create MnO2, to a reaction where we store Zn2+ and H+ ions inside MnO2 structures.
In a Cl rich electrolyte, we can likely get high CE at low capacities from the start, because the MnO2 reaction is more reversible than in a chloride-free environment. We shift to ion storage slowly with time, but we seemingly cannot do this at anything but really low capacity values.
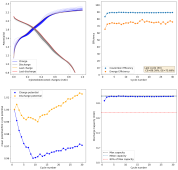
In a sulfate-only environment, the CE is lower if you do the same thing, but if you generate ample MnO2 by having a few cycles to much higher capacity than you intend to use - losing electrons intentionally to a less reversible MnO2 formation - you can then use the created MnO2 for ion storage.
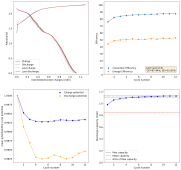
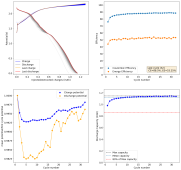
After cycling the battery 5 times to 2.6mAh, I was then able to get really high CE values to 1mAh. Something that I just cannot do in chloride containing electrolytes. Exp31a shows 54 charge/discharge cycles to 1mAh, after this initial priming process:
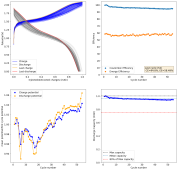
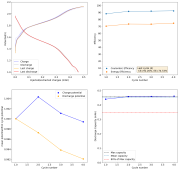
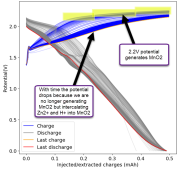
I then decided to hold the battery at 2.2V for 1 hour, to see if I could create more MnO2 and charge the battery to 1.5mAh. I could then get a discharge capacity of 1.4mAh, with a CE of 94% and an EE of 59% on the first cycle. Given this geometry and this capacity, this battery would be at 16Wh/L. We can likely take it higher with the proper preconditioning, given these observations.
This likely shows that the easiest path for these batteries to give decent capacity is to use a sulfate-rich electrolyte and perform some pre-conditioning process so that we can generate all the MnO2 we need, then cycle the battery through an intercalation process, avoiding reaching potentials significantly above 2V for any long period of time.
The trick is that this preconditioning needs to be carried out quite slowly, if you try to do it fast, then the strong overpotential heavily stimulates dendrite formation and you short the battery quite fast. Saturating the electrolyte with K2SO4 seems to increase the conductivity and ion migration issues enough to avoid this issue, at least in the short term. You also need to make sure not to go to high current densities, so I was careful to stay at or below 5mA/cm2.
I will let you know how cycling at 1.5mAh goes. I will also do some further preconditioning after that and try to go to even higher capacity.