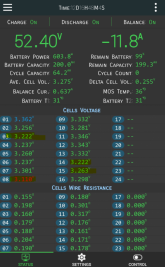
I'm finally in the process of building my first LifePo4 pack and I bumped into a really weird behavior that I can't quite explain.
If I compare voltages between screw terminals, I get reading A.
If I compare voltages between busbars (connected to the same screw terminal), I get a different reading B.
The difference is more than 0.1v (i.e 3.2v vs 3.34v)
I first noticed this in my BMS testing when it was reporting higher voltages than my voltage readings which I was doing against the screw terminals directly. Any idea why and is this normal?
If I compare voltages between screw terminals, I get reading A.
If I compare voltages between busbars (connected to the same screw terminal), I get a different reading B.
The difference is more than 0.1v (i.e 3.2v vs 3.34v)
I first noticed this in my BMS testing when it was reporting higher voltages than my voltage readings which I was doing against the screw terminals directly. Any idea why and is this normal?