totally agree! formatting makes it so hard to read and understand.
View attachment 82453
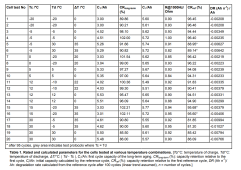
Tc is cell temperature while charging
Td is cell temperature while discharging
(people here often have these the same)
So mainly look at rows with DT/C of 0.
Ci is first reference cycle capacity at room temperature. note they are all almost the same of ~5.6 Ah
C1 is first cycle at the temperatures Tc,Td of row. note how cycles in cold temps have low capacity of down to 3.0 Ah at -20/-20 Tc/Td.
Note this capacity loss is reversible by bringing the cell back to room temperature.
CRref is the capacity AFTER all the cycles, but done at the reference temperature to remove the reversible temperature related capacity loss.
DR (Ah/N)Ah is derivation of how much total capacity is lost for a single cycle given the teat temperatures.
perfect battery will have same capacity for Ci,C1,CRref and would have DR of 0.00
hope this helps!
basically, for super fast reading,
choose your operating temperature. find closest match under Tc/Td. go all the way to the right to see DR or the fraction of capacity lost per cycle at those temps.