LOL that's funny because I went to page 60 on my PDF Prg and it was actually page 44 of Document.
The very first thing I read is this:
_______________________________________
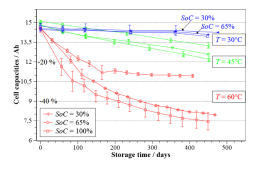
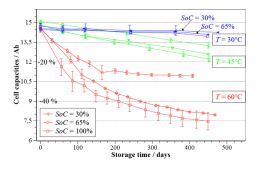
4.2 Analyzing cells stored at 100% SOC at different temperatures
The two variables that most affect degradation within Li-ion cells under OCP conditions are
temperature and SOC. [9] The most predominant cause of degradation under OCP is the storage
temperature of the cell. When the storage temperature for the cell is high, secondary reactions
proliferate causing accelerated losses of the cyclable lithium (the main source of losses within
the cell). [6] Testing of various cells at different SOCs but under the same temperature storage
45
conditions has, also, led to the determination that SOC plays a major role in cell degradation.
[14] Cells stored at elevated SOCs experienced increased battery degradation compared to those
stored at lower SOCs [61]. SOC represents the proportion of ions present on either electrode,
thus, for high SOC there is a significant number of lithium-ions available at the graphite
electrode to partake in potential side reactions with the electrolyte.
In addition to the irreversible capacity losses that can occur at high SOC, the degree to which
reversible capacity will occur is also affected. In comparison to the irreversible losses that can
occur within the cell, reversible losses were found by Safari and Delacourt to be more affected
by SOC than by temperature. [14] Li-ion batteries do experience the lowest reversible capacity
losses.
--------------------------------------------------------------------------------
Once again like in every other document I have seen they talk about it as being a Lithium issue and then separate out Lithium Ion as actually having less of a problem than other Lithium chemistries.
I have to leave for a bit but will pick this back up later when I get back home.