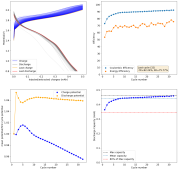
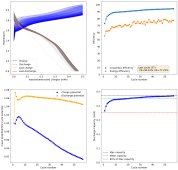
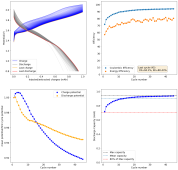
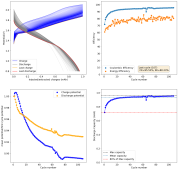
danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
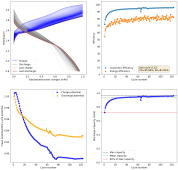
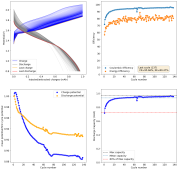
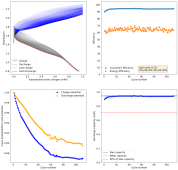
Daniel, do you have any new results?
No, I moved countries again. This time I moved from Colombia to Spain, so I had to leave all my battery experiment stuff in Colombia with family. I will hopefully get the electronic and electrode parts back when they come to Spain later this year.