@Skypower @RCinFLA and other, thanks for your response.
Concerning the YR1035+ tester, it is clearly written in the doc that it is not precise below 0.3 mOhm. So measuring a single cell or a bus bar is not precise but as Skypower said it gives a reference.
On the other hand, measuring the resistance of the complete pack should give a value close to reality.

Concerning the cells provided by QSO with the glossy terminal without helicoil.
I assembled this pack for the first time with the bus bars (non-flexible) delivered with the cells and 3 months later I assembled this pack in a box with flexible bus bars as in the photos below. The only thing I did was scrub the terminals and bus bars with 96% pure alcohol before assembling them.

In both cases, I measured the IR for the entire pack between the "-" and "+" terminal (before connecting the BMS).
Here are the results :
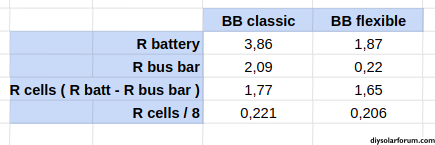
- QSO 8S pack with classic bus bar: 3.86 mΩ
- QSO 8S pack with flexible bus bar: 1.87 mΩ
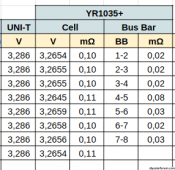
To understand the table below, the measurement of the IR of the bus bars between the cells (0.02 mΩ) was made between the "+" cell 1 and the "-" cell 2 from the EVE unwelded terminal as indicated by the pink line in the photo below. This is to take into account the entire bus bar including the welded terminal.

Below is my measurement table with the flexible bus bars.
Based on the total internal resistance of the battery and the bus bars I tried to estimate the "real" internal resistance of the cells. (value in mΩ)
All this without having rubbed the termials with “Scotch-Brite” or applying NO-OX-ID...
So the big question remains, is it really necessary to sand with “Scotch-Brite” and put NO-OX-ID ?














