danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
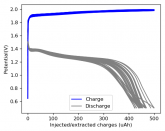
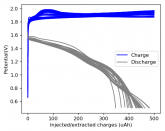
The voltage drop above reminds me more of the behavior of a capacitor than a cell. For example with LiFePO4 there's a nominal voltage, similar to how the temperature of water holds steady at 0° C as it freezes.
I take it that at 1 mA what you're really measuring is the ion flow through the electrolyte?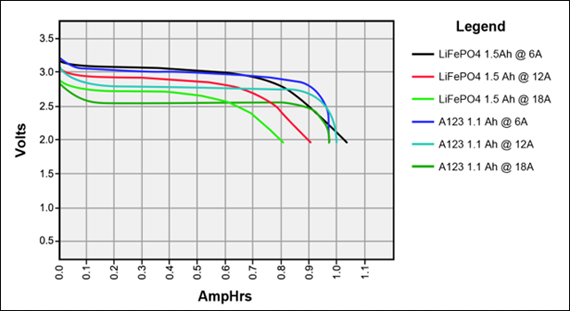
I measured the capacitance of the cells using cells without ZnBr2. Their capacity due to capacitance is just around 1uAh, which is reasonable given the electrode separation and dielectric constant of the solution.The charge extracted here is more than 30x that, so it is due to a chemical process. When I take charged cells apart I can see the plated Zn and deposited TBABr3 so the chemistry is definitely happening.
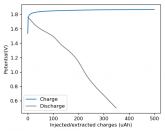
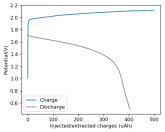
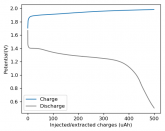
Some batteries have strong potential plateaus while others have more linear downslopping curves. This depends on how well the battery can sustain the chemical reactions through the discharge phase at the current density demanded by the discharge process.
The CC6 carbon electrode is bad at this, possibly because of a much lower surface area and conductivity, but the process is no doubt based on the chemistry of the battery.
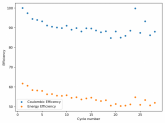
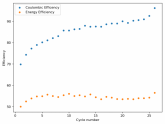
The discharge at 1mA is not limited by ion flow through the battery but most likely by the kinetics of TBABr3 reduction in the CC6 electrode. Self-discharge is likely to also be an important factor limiting efficiency at these charge/discharge currents.
Last edited: