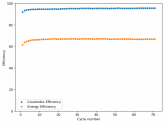
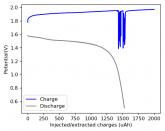
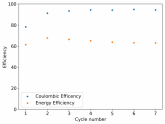
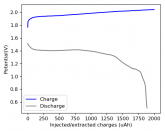
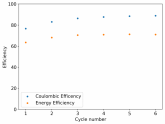
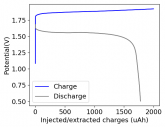
So I decided to take apart this battery after 11 cycles. Here's the final result:
Final curve has a CE=86.41% and an EE=68.74%.
The reason why I took it apart is because there started to be some deterioration of the cell. The coulombic efficiency wasn't sufficiently high and the cycles were taking too long. Plus I have only one Swagelok cell and I wanted to perform an interesting test today since I had the time to put together another cell before the start of the week

Given how the solubility of quaternary ammonium bromides drops as a function of ZnBr2 concentration, it is likely unnecessary to pre-saturate the ZnBr2 solution with TMPhABr before putting it in the cell, especially if we include a high enough amount of the solid within the cell. The idea is then to move to what the ideal concentration of ZnBr2 in a high density battery would be (around 3M + 1% PEG-200) and see if we can just add that to the battery if we include a 50mg TMPhABr layer between the CC4 cathode and the fiberglass layers.
I noticed that after closing the Swagelok cell the TMPhABr solid layer is compacted quite nicely between the cathode and the fiberglass layer, so I decided to close the cell before adding the electrolyte - to compact it - then open it up again to add the electrolyte, waiting until 100uL soaked entirely through the cathode and the fiberglass layers (around 2 minutes).
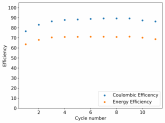
I have built a battery per the above in my Swagelok cell now. I am going to try to charge this to 5000 uAh at 5mA, 10x the specific energy of the initial cells I was testing. If this is achieved, the battery will be above the specific energy of lead acid and close to the upper part of the range of commercial flow Zn-Br batteries. Will we achieve this amazing feat??

(probably not on the first try

)