danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
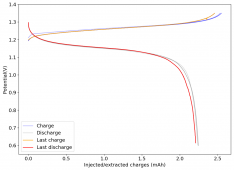
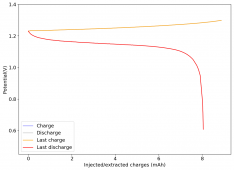
The first WiS (water-in-salt) battery I constructed dried due to some air openings in my Swagelok cell. I added teflon tape to the electrodes to provide a tight seal and prevent contact with the outside environment. I then made a second WiS Zn-I device per the description in the post linked in #24. This is the first cycle:
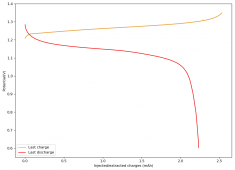
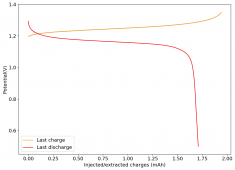
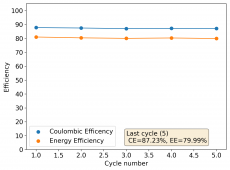
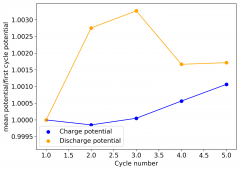
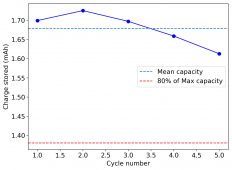
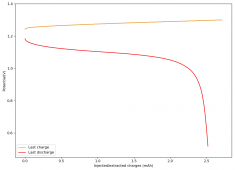
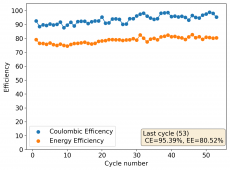
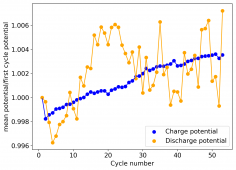
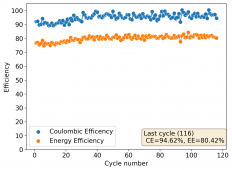
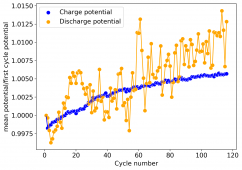
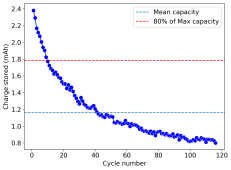
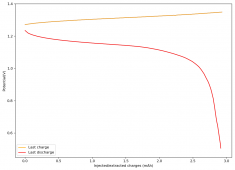
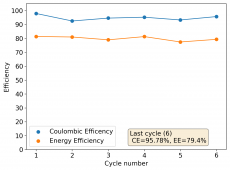
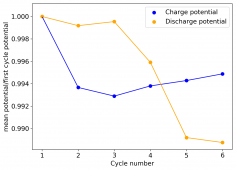
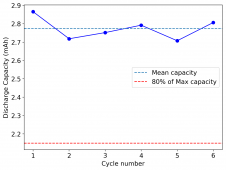
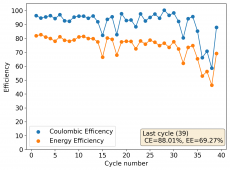
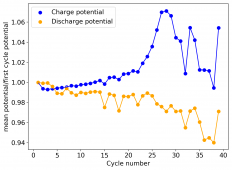
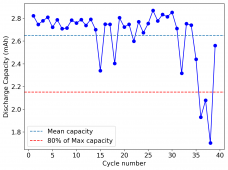
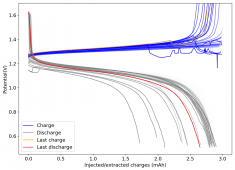
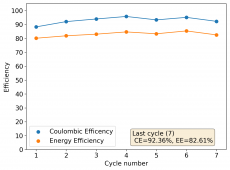
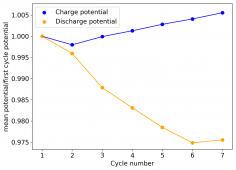
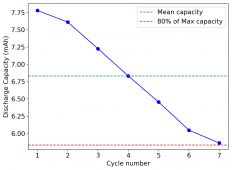
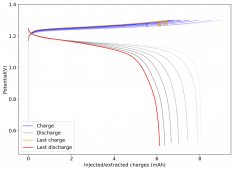
The device was charged to 1.35V and discharged to 0.6V at a current of 5mA (3.87mA/cm2). This first cycle showed a CE of 88% with an EE of 78%. The measured capacity is 2.23mA/h, given the dimensions of the device, this gives an energy density of 62 Wh/L. I will keep cycling it and post some more results.
This is the best device I have ever built! ?

The device was charged to 1.35V and discharged to 0.6V at a current of 5mA (3.87mA/cm2). This first cycle showed a CE of 88% with an EE of 78%. The measured capacity is 2.23mA/h, given the dimensions of the device, this gives an energy density of 62 Wh/L. I will keep cycling it and post some more results.
This is the best device I have ever built! ?
Last edited: