danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
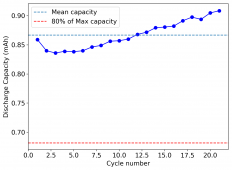
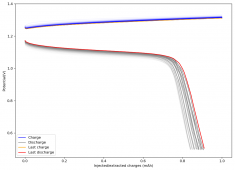
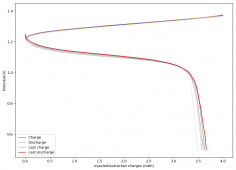
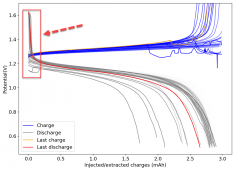
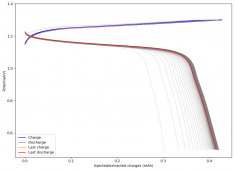
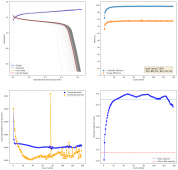
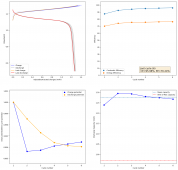
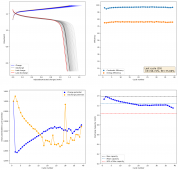
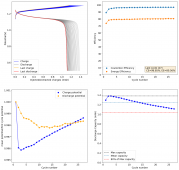
For these tests, I built a battery using the 15m ZnCl2, 5m KI, 5m NaCl WiS electrolyte using a CC6P cathode and two layers of fiberglass. I decided to charge the battery to just 1mAh of fixed charge capacity, at 10mA. This is in order to test cycling stability quickly as I have always been getting degradation as a function of cycle number, even at lower capacities.
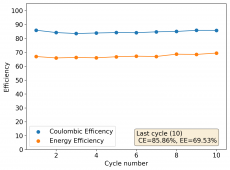
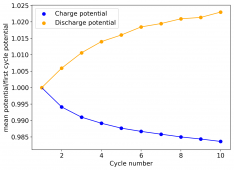
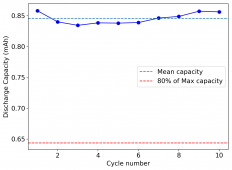
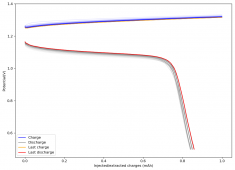
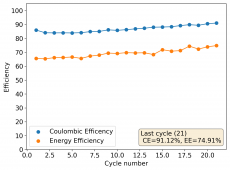
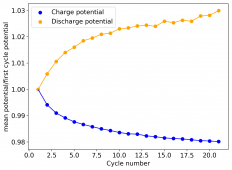
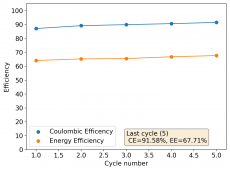
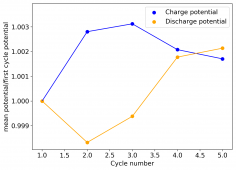
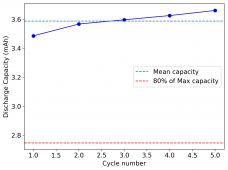
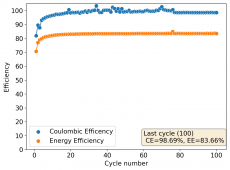
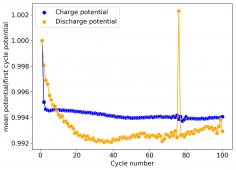
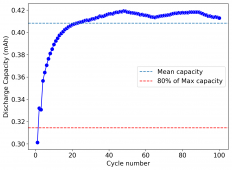
I am very excited to say that after 10 cycles, the capacity has been steady and the charge/discharge voltages have actually improved as a function of time. The coulombic and energy efficiency values did decrease a little bit compared to previous experiments without NaCl in the WiS electrolyte, but stability has increased tremendously. I am going to cycle 50 times to this capacity, if stability remains good, I will proceed to cycle at higher capacities (4mAh) and see what we get ?




I am very excited to say that after 10 cycles, the capacity has been steady and the charge/discharge voltages have actually improved as a function of time. The coulombic and energy efficiency values did decrease a little bit compared to previous experiments without NaCl in the WiS electrolyte, but stability has increased tremendously. I am going to cycle 50 times to this capacity, if stability remains good, I will proceed to cycle at higher capacities (4mAh) and see what we get ?