danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
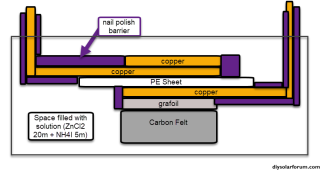
For the first experiments I have been using Cu anodes (easily cut from copper tape), which have been easy to adhere to the Titanium electrodes and sand to reveal pristine copper. I also sanded down the Ti electrodes (320 and then 240 papers) before these experiments to ensure they are much more flat as they previously had some small irregularities due to the way in which they were cut.
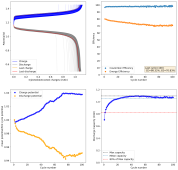
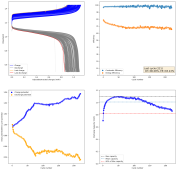
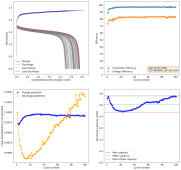
The results so far have been really good, without any dendrites, when using a fiberglass separator. I was even able to run a cell for 141 cycles at 10mA/cm2 with a single layer of fiberglass separator with no issues (Exp 3b).
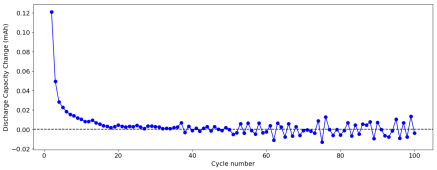
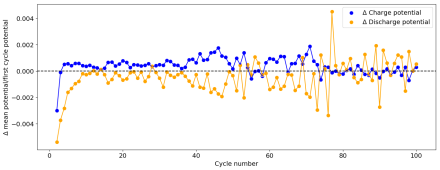
However, I am still experiencing substantial capacity decay. The fact that the decay is slower at higher current (10 mA/cm2 Vs 5 mA/cm2) (Exp 3a and 3b), suggest that the decay is related to some species that are generated and diffuse away from the electrodes. Since there is less time for diffusion at higher current, you expect diffusion related decay to be less prominent as electrode cycling becomes faster. This also matches the fact that Coulombic efficiency increased with current (97 at 10 mA/cm2 Vs 93 at 5mA/cm2).
I am now running an experiment (Exp 4) including 1M NaCl in the solution, to see the effect of further reduced water activity. After this is done I will run additional experiments at 2M NaCl and 5M NaCl, to see the effect this additive has on cycling characteristics.
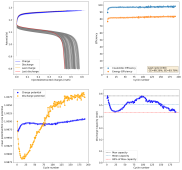
The results so far have been really good, without any dendrites, when using a fiberglass separator. I was even able to run a cell for 141 cycles at 10mA/cm2 with a single layer of fiberglass separator with no issues (Exp 3b).
However, I am still experiencing substantial capacity decay. The fact that the decay is slower at higher current (10 mA/cm2 Vs 5 mA/cm2) (Exp 3a and 3b), suggest that the decay is related to some species that are generated and diffuse away from the electrodes. Since there is less time for diffusion at higher current, you expect diffusion related decay to be less prominent as electrode cycling becomes faster. This also matches the fact that Coulombic efficiency increased with current (97 at 10 mA/cm2 Vs 93 at 5mA/cm2).
I am now running an experiment (Exp 4) including 1M NaCl in the solution, to see the effect of further reduced water activity. After this is done I will run additional experiments at 2M NaCl and 5M NaCl, to see the effect this additive has on cycling characteristics.