danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
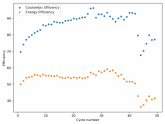
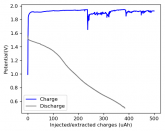
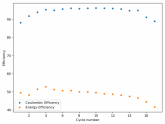
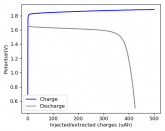
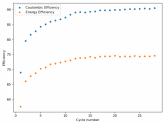
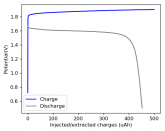
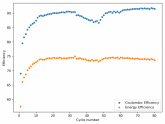
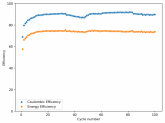
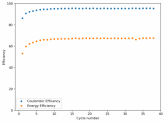
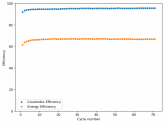
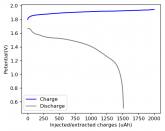
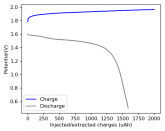
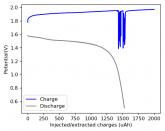
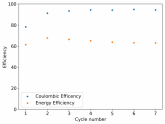
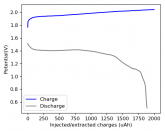
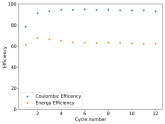
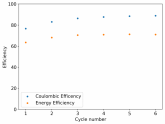
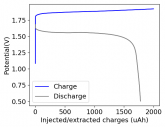
In my research of Zinc-Bromine battery literature I also discovered that Robert Murray Smith filed an application for a patent for an improved electrolyte for a Zinc-Bromine battery where the sequestering agent is made of - the most British thing ever - black tea (https://worldwide.espacenet.com/pub...T=D&ND=3&date=20191211&DB=EPODOC&locale=en_EP). The theory is that the polyphenols in black tea will serve as sequestering agents. The patent does contain some summarized experimental data, although no charge/discharge data or stability data is given. Without detailed data, it is hard not to be skeptical of these results or whether these batteries have any meaningful cycle life.

I strongly doubt this works to build a stable battery (as described) because polyphenols would not be present in either high enough concentrations or offer reversible enough chemistry to be useful for this purpose. Plus, there are a lot of compounds in black tea that will react irreversibly with elemental bromine.
This is a patent though, so the application might be misleading on purpose and they might be using a specific pure polyphenol - which might be present in black tea and grape juice - as a sequestering agent (meaning he would just be using this odd black tea description to protect the IP). Definitely makes me curious about this chemistry.

I strongly doubt this works to build a stable battery (as described) because polyphenols would not be present in either high enough concentrations or offer reversible enough chemistry to be useful for this purpose. Plus, there are a lot of compounds in black tea that will react irreversibly with elemental bromine.
This is a patent though, so the application might be misleading on purpose and they might be using a specific pure polyphenol - which might be present in black tea and grape juice - as a sequestering agent (meaning he would just be using this odd black tea description to protect the IP). Definitely makes me curious about this chemistry.
Last edited: