danielfp248
Battery researcher
- Joined
- Sep 7, 2020
- Messages
- 429
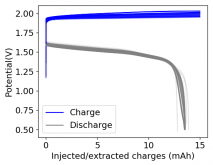
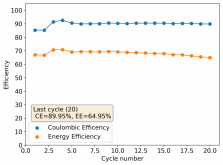
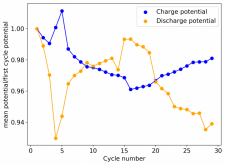
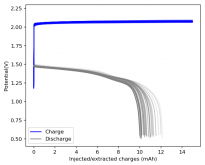
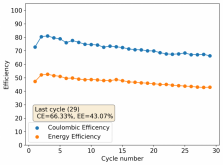
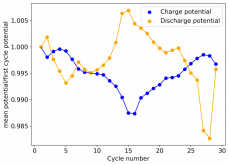
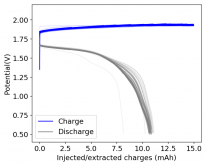
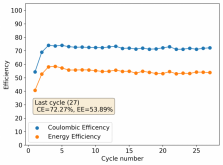
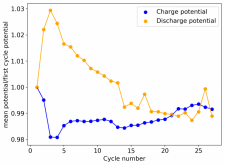
Finally there was a very high increase in the potential required for charging and a big drop in the discharge potential during the 24th cycle. Reason why I stopped cycling the battery at this point.
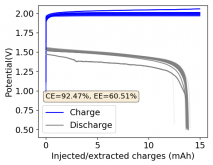
It is interesting though that this is not the characteristic failure due to zinc dendrites, where there are sudden and sharp declines in the potential due to the dendrites touching the cathode. Opening up the cell revealed no dendrite formation, although there were a significant amount of crystals formed at the edges of the fiberglass separator. This indicates that the problem might have actually been electrolyte losses, possibly due to evaporation.
I have now put together a separator-cell less using 3 PTFE o-ring spacers - which gives me the same total cell volume - to see if we get a similar result with this 1% PEG-200 + 1% Tween 20 + 3M ZnBr2 electrolyte when no separators are used.

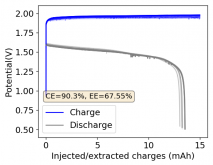
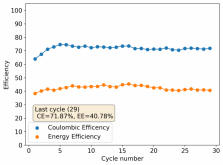
It is interesting though that this is not the characteristic failure due to zinc dendrites, where there are sudden and sharp declines in the potential due to the dendrites touching the cathode. Opening up the cell revealed no dendrite formation, although there were a significant amount of crystals formed at the edges of the fiberglass separator. This indicates that the problem might have actually been electrolyte losses, possibly due to evaporation.
I have now put together a separator-cell less using 3 PTFE o-ring spacers - which gives me the same total cell volume - to see if we get a similar result with this 1% PEG-200 + 1% Tween 20 + 3M ZnBr2 electrolyte when no separators are used.